Maple Syrup Urine Disease (MSUD)
Table of Contents
What is a Maple Syrup Urine Disease?
Maple Syrup Urine Disease (MSUD) is a rare genetic disorder characterized by the body’s inability to process certain amino acids properly. The condition gets its name from the sweet, distinctive odor of affected infants’ urine, which resembles that of maple syrup.
This metabolic disorder primarily impacts the way the body breaks down the amino acids leucine, isoleucine, and valine, which are present in protein-containing foods. MSUD can lead to a dangerous buildup of these amino acids and their byproducts, resulting in severe health complications if left untreated.
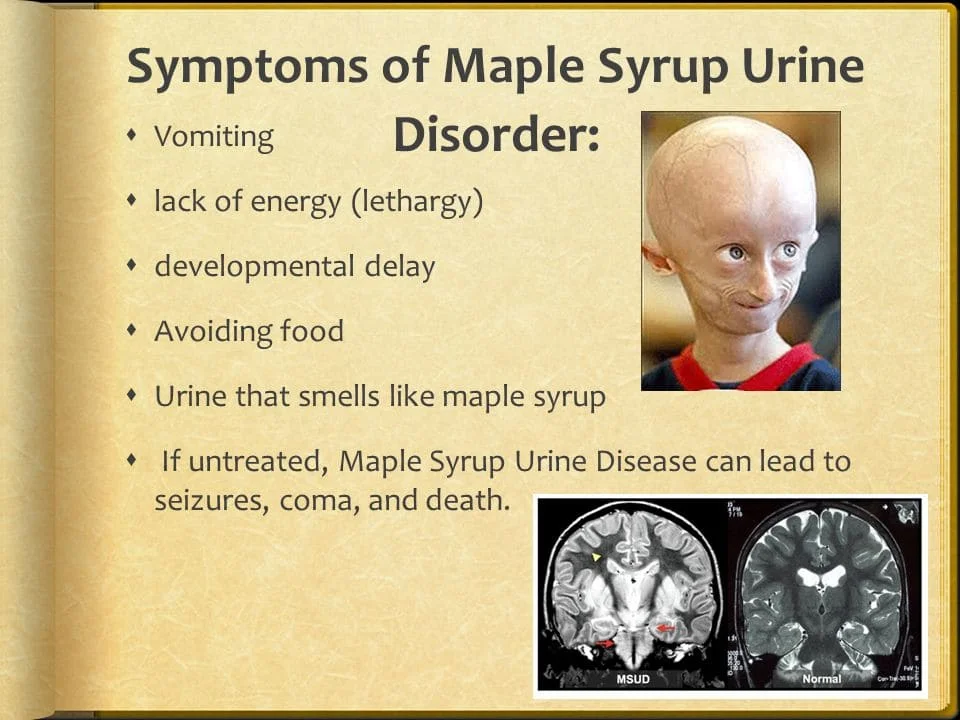
Symptoms of MSUD can include feeding difficulties, irritability, lethargy, distinctive sweet-smelling urine, and neurological problems such as developmental delays, seizures, and even coma. Early diagnosis and prompt management are crucial to prevent long-term complications and ensure a better quality of life for individuals affected by this disorder.
Treatment typically involves strict dietary management, which aims to regulate the intake of certain amino acids, and in some cases, supplementation of specific formulations to ensure balanced nutrition. Early intervention and ongoing medical support play a critical role in managing MSUD and mitigating its potential health risks.
Introduction
In 1954, Menkes’ neurodegenerative illness—now known as maple syrup urine disease (MSUD)—was initially characterized as having a sudden onset. It is a metabolic deficiency brought on by the branched-chain alpha-ketoacid dehydrogenase (BCKAD) complex’s aberrant activity. Branch-chain amino acid degradation is brought on by this complex:
- Leucine
- Isoleucine
- Valine
Branch-chain amino acid (BCAA) buildup in the plasma and corresponding branched-chain ketoacids in the urine are caused by the underlying deficiency in the BCKAD complex, which interferes with the metabolism of branched-chain amino acids.
It typically shows up in the newborn period as failure to thrive, missed developmental milestones, feeding issues, and a urine or cerumen odor that smells like maple syrup. Branch-chain amino acid restriction and thorough metabolic monitoring make up the course of treatment. A metabolic catastrophe and irreparable cerebral damage result from untreated conditions. Optimal clinical results can be anticipated with early management.
Causes
The multienzyme complex of BCKAD is made up of four subunits. These subunits contain BCKAD decarboxylase (E1 subunit) coupled to dihydrolipoyl acyltransferase (E2) and dihydrolipoamide dehydrogenase (E3 subunit). The core of the E2 subunit is additionally connected to two regulatory subunits, BCKAD kinase and phosphatase.
Additionally, the E1 subunit is made up of two E1beta and one E1alpha subunit. Reduced activity of the mitochondrial branched-chain alpha-ketoacid dehydrogenase (BCKAD) complex is the cause of the metabolic disease. The inheritance pattern for the condition is autosomal recessive. There are five main clinical phenotypes of maple syrup urine disease.
Epidemiology
Males and females are equally affected by maple syrup urine illness. One case per 185,000 live births is the estimated incidence rate for the entire world.[1] There have been more cases reported in groups with higher consanguinity rates. According to estimates, the incidence occurs in 1 in 26,000 live births among Ashkenazi Jews. In Mennonites, the prevalence of maple syrup urine illness is 1 in 380 births. In the BCKDHA (E1a) gene (c.1312T>A), this is frequently referred to as a founder’s effect. 1.4% of cases in Portuguese gypsies are caused by the homozygous deletion of the BCKDHA gene (117delC). One instance is thought to occur for every 71 births.
Pathophysiology
- In many tissues, including skeletal muscle, the liver, the kidney, and the brain, branched-chain ketoacid dehydrogenase (BCKAD) is found within the inner mitochondrial membrane.
- E1, E2, and E3 are the three catalytic subunits that make up this unit. It aids in mediating the catabolism of branched-chain amino acids (BCAA) along with branched-chain amino acid transaminase.
- Thiamin pyrophosphate causes E1 to decarboxylate the alpha ketoacids.
- The acyl group from E1 is transferred to CoA via the lipoic acid residue in E2. The lipoic acid residue in E2 is reoxidized with aid from the E3 subunit. BCKAD phosphatase and BCKAD kinase further influence the activity of branched-chain ketoacid dehydrogenase.
- Therefore, the enzyme branched-chain amino acid transaminase converts branched-chain amino acids into their corresponding alpha-ketoacids inside the mitochondria.
- Alpha-ketoisocaproic acid, alpha-keto-beta-methyl valeric acid, and alpha-ketoisovaleric acid are among the alpha-ketoacids that they each produce.
- The branched-chain ketoacid dehydrogenase complex then performs oxidative decarboxylation on the alpha-ketoacids.
- Alpha-ketoacids are therefore converted into intermediates such as isovaleryl-coenzyme A, alpha-methylbutyryl-CoA, and isobutyrl-CoA during subsequent metabolism.
- Then succinyl-CoA, acetoacetate, and acetyl-CoA are produced from these intermediates.
- The hydrophobic side chains of the branched-chain amino acids, which are abundant in foods high in protein, make them necessary amino acids.
- These amino acids must be catabolized in order to maintain a number of physiologic processes, including:
- Synthesis of proteins
- Gluconeogenesis
- Synthesis of fatty acids
- cholesterol production
- cellular communication
- BCKAD aids in the metabolism of BCAA in the brain, facilitating the synthesis of GABA and glutamate in the brain.
- 10% to 15% of the BCAAs are catabolized by the liver and kidney. Skeletal muscle is where most BCAA transamination and oxidation takes place.
- A pathogenic deficiency in any BCKAD subunit causes maple syrup urine disease, which is characterized by high levels of branched-chain amino acids and their related alpha-keto acids.
- Alpha-ketoacids and accumulated BCAA cause skeletal muscle, the immunological system, and the central nervous system to malfunction, which results in a constellation of clinical symptoms.
- Leucine and alpha-ketoisocaproic acid levels that are elevated are well known to disrupt neurochemical processes and result in clinically obvious neurotoxicity.
- Because of leucine’s interference, big neutral amino acid transport through the blood-brain barrier is significantly diminished.
- Tyrosine, phenylalanine, methionine, tryptophan, histidine, and valine are consequently supplied to the brain.
- As a result, myelin synthesis and brain growth are adversely affected.
- Reduced levels of neurotransmitters like dopamine, serotonin, norepinephrine, epinephrine, GABA, and glutamate are caused by the limited availability of amino acids.
- More than 60 micromol/L of alpha-ketoisocaproic acid negatively controls transamination processes in astrocytes.
- Low levels of brain glutamate, which cause cognitive dysfunctions such as learning difficulties and memory loss, are explained by this reversal.
- Furthermore, the regulation of cell volume is hampered by high leucine concentrations.
- This causes a drop in blood osmolarity, a rise in intracellular water, and a concentration of sodium that causes cerebral edema.
- Reduced blood osmolarity can hasten brain herniation in babies and young children.
- The neurotoxic alpha-ketoisocaproic acid may play a role in the encephalopathic condition, according to clinical findings.
- Lower glutamate levels and higher cerebral lactate levels are signs of respiratory chain inhibition caused by alpha-ketoisocaproic acid in patients with classic MSUD. The blood-brain barrier breaks down as a result of the metabolic decompensations in MSUD-activating matrix metalloproteinases. The metabolites of isoleucine are what give urine its maple syrup smell.
- Physical and historical
- The clinical subtypes of MSUD are five. These consist of:
- Types of the sickness caused by maple syrup urine
- There are four primary categories of MSUD:
- The most severe kind is called classic maple syrup urine illness. It’s the most typical as well. Typically, within the first three days following birth, symptoms appear.
- Intermediate: Compared to classic MSUD, this type is less severe and symptoms typically manifest between 5 months and 7 years.
- Children with intermittent MSUD grow and develop as predicted up until an infection or stressful time when symptoms start to show. In contrast to people with classic MSUD, those with intermittent MSUD typically tolerate higher doses of the three amino acids.
- Thiamine-responsive: This kind of MSUD is treated with a restricted diet and large doses of vitamin B1 (thiamine). Patients with thiamine-responsive MSUD who receive treatment exhibit greater tolerance for the three amino acids.
- How common is maple syrup urine disease (MSUD)?
- Rarely does MSUD occur. One in every 185,000 kids born around the world is impacted by it.
- When close relatives have children together or in societies with tiny gene pools, it happens more frequently. For instance, Mennonites in the United States, where community members frequently marry and have children, have a higher incidence of MSUD (1 in every 380 births).
- Additionally, it occurs more frequently among Ashkenazi Jews (1 in every 26,000 births).
- Clinical features such as age of onset, clinical presentation, and residual BCKAD enzyme activity allow for clinical differentiation.
- The connection between an individual’s genetic makeup and their observable characteristics is not always clear.
- The BCKAD residual function is necessary for the clinical presentation.
- The way an organism responds to metabolic decompensation and leucine tolerance, however, determines its phenotypic classification.
Evaluation
The evaluation of maple syrup urine disease necessitates the quick identification of clinical traits by a medical professional. Evaluation of the disease phenotype also heavily weighs biochemical and molecular testing in addition to clinical characteristics.
Symptoms
The clinical aspects of MSUD are influenced by its underlying metabolic abnormality. The following manifestations should be taken into consideration:
- Within the first 12 hours after delivery, the first clinically discernible symptom is the smell of maple syrup in the cerumen.
- Two to three days old irritability, ketonuria, and inadequate nutrition
- A deteriorating encephalopathy that manifests as apnea, opisthotonus, and fatigue.
- Furthermore, by the age of 4 to 5 days, movements for fencing and cycling might also develop.
- By the age of 7–10 days, coma and respiratory failure have set in.
Less severe variations of the illness may manifest as anorexia, stunted growth, and delayed developmental milestones later in infancy or childhood. Encephalopathy and ketonuria in such circumstances will only occur after metabolic decompensation during an acute illness.
Diagnosis
If any of the following apply to children or newborns, they should be evaluated for MSUD:
- encephalopathy sporadic and ketoacidosis
- sporadic encephalitis with ketoacidosis
- Ketoacidosis and encephalopathy associated with an underlying disease
- encephalopathy and ketoacidosis after fasting or trauma
- ketoacidosis and encephalopathy combined with a negative neonatal screening result
Prenatal Diagnosis
Utilizing mutational analysis, the diagnosis necessitates measuring the BCKAD enzyme activity in cultivated chorion villus cells or amniocytes. Amniotic fluid can also be used to test the amounts of branched-chain amino acids. Finding family pathogenic variations is necessary for pre-implantation diagnosis. The molecular analysis method is the preferred diagnostic approach.
Screening and Biochemical Assessment of Newborns
- Newborns have been routinely screened for MSUD since 1964.
- All fifty states in the union conduct the screening, which is best done 24 to 48 hours after birth. Tandem mass spectrometry amino acid profiling (MS/MS) is used to carry it out.
- As a conventional measure, the procedure looks at leucine-isoleucine concentrations and the fisher ratio.
- If there is evidence of higher branched-chain amino acids, more laboratory research must be done. Classic MSUD can be easily detected with tandem mass spectrometry.
- However, because of normal leucine levels, it might miss milder instances of MSUD. Amino acids with the same mass, such as hydroxyproline, leucine, isoleucine, and also-isoleucine, cannot be distinguished by MS/MS.
- In these situations, allo-isoleucine on dried blood spots needs to be analyzed using a second-tier technique, like liquid chromatography.
- Hydroxyprolinemia and MSUD can be distinguished with the aid of second-tier tests.
- The dinitrophenylhydrazine test, gas and liquid chromatography, BCKAD enzyme activity, and molecular testing are further laboratory investigations.
- For MSUD, plasma amino acid testing is the most crucial diagnostic procedure.
- It is employed to evaluate high allo-isoleucine and BCAA levels. High amounts of allo-isoleucine (> 5 micromols/L) provide excellent sensitivity and specificity for diagnosis.
- Allo-isoleucine levels are only noticeable at six days of age, even with increased leucine levels.
- Urine test strips, dinitrophenylhydrazine assays, and gas chromatography-mass spectrometry can all be used to identify the organic acids found in the urine.
- Branching-chain ketoacids are found by gas chromatography-mass spectrometry, which adds more evidence to the MSUD diagnosis. Branched-chain ketoacids are often seen 48–72 hours after delivery and appear after an increase in BCAAs.
- Branching-chain ketoacids in the urine can also be found using the dinitrophenylhydrazine (DNPH) test.
- Urine and DNPH reagents are combined in equal parts.
- After that, the sample is watched for ten minutes to look for any color changes or precipitation. A score of 0 is indicated by precipitate-free, clear urine.
- On the other hand, a score of 4 is indicated by a yellow-white precipitate and opaque urine.
- The DNPH test has the benefit of being able to identify urinary branched-chain ketoacids in an outpatient environment.
- Ketone urine can be detected using regular test strips. When access to other diagnostic tests is restricted, it provides an alternate testing source.
- It is said that ketonuria serves as a stand-in indicator for underlying metabolic instability.
- It is possible to quantify the BCKAD enzyme activity in cells including skin fibroblasts, lymphoblasts, and liver biopsy samples.
- Because leucine tolerance and oxidation do not correlate with BCKAD activity levels, in vivo measures of this enzyme’s activity are not clinically useful.
Molecular examination:
For three biallelic variations of the pathogenic gene, molecular testing is available. These consist of:
- The BCKAD enzyme complex’s E1-alpha component (MSUD Type 1A) is encoded by the BCKDHA gene.
- The BCKAD enzyme complex’s E1-beta component (MSUD Type 1B) is encoded by the BCKDHB gene.
- The DBT gene genes for the BCKAD enzyme complex’s E2 component (MSUD Type 2).
- Better knowledge of the prognosis and genetic counseling for the family is made possible by genetic testing. Moreover, it permits precise evaluation of the defective BCKAD component.
- This aids in deciding on customized treatments.
- The BCKAD enzyme subunits have over 190 pathogenic variants. Every variation found is either compound heterozygous or homozygous.
Diagnostic Techniques
An adult exhibiting MSUD symptoms:
- Alpha-ketoacids in the urine can be detected by DNPH testing.
- The test that provides the greatest information is the plasma amino acid analysis test for allo-isoleucine identification.
- Gas chromatography-mass spectrometry may identify additional organic acids as well as branch-chain ketoacids.
- A newborn exhibiting symptoms and a positive screening test result for valine, leucine, and isoleucine, or an inexplicable case of ketonuria:
- It is possible to employ screening tests such as urine ketone test strips and DNPH if the infant is older than 48 to 72 hours.
- Analyzing plasma amino acids can reveal higher levels of allo-isoleucine and BCAA.
- Urine is subjected to gas chromatography-mass spectrometry analysis to check for ketoacids.
- It is not advisable to challenge newborns and infants with higher-than-normal protein intake.
- A newborn whose sibling is impacted:
- Melting analysis, polymerase chain reaction (PCR), and sophisticated sequencing can all be utilized to find pathogenic variants in isolated cord blood if familial variants are known.
- Get blood from the umbilical cord to enable pathogenic variant detection if the pathogenic variants are unknown. A newborn’s cerumen smelling like maple syrup is the first diagnostic indicator.
- Therefore, 12 to 24 hours after birth, ask if there is a smell like maple syrup.
- Allocate 18 to 24 hours of life for the measurement of serum amino acid levels based on protein intake. Repeat the test after 24 to 36 hours of life if the results are not entirely clear.
- Dietary therapy should be started if the amino acid profile is suggestive of MSUD. Urine organic acid analysis and molecular testing ought to come next.
- It is crucial to keep in mind that DNPH cannot be utilized as a screening test prior to the 48–72 hour mark.
Differential diagnosis
Excluding other clinically different diseases that may also present as newborn encephalopathy is crucial. These consist of:
- Low blood sugar
- Epileptic status
- Diarrhea
- Incapacitation
- A. Kernicterus
- natal asphyxia
- Enzyme cycle flaws
- organic acidophiles, including methylmalonic acidemia and propionic academia deficit of HMG-CoA lyase
- Lack of beta-keto thiolase
- Hyperglycemia that is not ketotic
- The substance sotolone, which may be found in fenugreek and lovage, is what gives cerumen and body their distinctive maple syrup smell.
- Overconsumption of fenugreek during pregnancy may lead to an incorrect MSUD diagnosis. Topical benzoin usage can also produce a nice smell in NICU settings.
Treatment
In order to treat maple syrup urine illness effectively, dietary deficiencies must be addressed and acute metabolic decompensations must be adequately managed. When managing patients with MSUD, a pediatric dietitian and metabolic disease specialist should be included.
Nutritional Therapy for Medicine
A favorable result from the newborn screening and clinical confirmation are needed before nutritional therapy can be started. The dietary limitation of branched-chain amino acids is still the cornerstone of treatment. These dietary adjustments must be kept up throughout life. The following are the objectives of nutritional therapy:
- Encourage anabolism
- Stop the catabolism
- Encourage healthy development and weight increase
- Maintain Cognitive Function
- Allow branched-chain amino acid limitation in the diet to assist in lowering harmful metabolites.
- Keep the levels of plasma BCAAs within the prescribed therapeutic parameters.
- Assess the responsiveness to thiamine.
- Growth measures made during the corresponding life phases and biochemical lab values are used to titrate the permitted amounts of dietary BCAA into the diet.
- Accurate calorie needs assessment, BCAA restriction, BCAA-free amino acid supplementation, and supplementation with valine and isoleucine are necessary for long-term treatment.
- Up to 80%–90% of required protein can be obtained from medical foods free of BCAAs. Isoleucine and valine aid in the promotion of anabolism.
- Due to their low content in medical nutrition, they are supplemented.
- As leucine is present in sufficient quantities in breast milk and formula, supplementing is typically not necessary.
- Healthy brain development can be attained by keeping leucine concentrations between 75 and 300 micromol/L.11] Maintaining plasma levels of isoleucine and valine between 200 and 400 micromol/L is also advised.
- Patients with homozygous 1312T>A mutations or those with mutations resulting in less than 3% of BCKAD activity shouldn’t take supplements of thiamine.
- Plasma osmolarity and serum sodium concentrations may be maintained with the administration of sodium chloride. It can lessen the chance of a deadly herniation or the onset of cerebral edema.
Acute Metabolic Decompensation Treatment
- The most common causes of metabolic decompensations (plasma leucine >380 micromol/L) are infections and non-compliance with diet.
- Dietary noncompliance increases the levels of BCAAs and infrequently leads to encephalopathy and decompensation.
- On the other hand, severe protein catabolism brought on by trauma and infections might result in a metabolic crisis.
- Decompensations are more likely after the age of fifteen and during the first year of existence.
- According to Strauss et al., the most frequent reasons for hospitalization during acute decompensation are vomiting and viral gastroenteritis.
- Neonatal encephalopathy, sinusitis, viral bronchiolitis, and urinary tract infections are more frequent reasons for hospitalization.
- The risk of a metabolic crisis is determined by leucine released from catabolism and residual BCKAD activity. Leucine tolerance is better in patients with more residual BCKAD activity when they are well.
- Moreover, these people experience less severe rises in leucine during an illness.
- Suppressing protein catabolism and promoting protein synthesis is the primary goal of treatment.
- In more serious situations, management techniques include:
- addressing the underlying stressor that is generating the metabolic crisis successfully
- Limit your protein intake for a full day or two.
- Ensure sufficient calorie intake
- Drink enough water to keep your metabolism in balance.
- Provide cofactors as a supplemental resource.
- Get rid of harmful metabolites
- Address any related clinical aftereffects
- rectify aberrant metabolic patterns
Rehab at Home
- The use of a dinitrophenylhydrazine reagent by providers to identify elevated levels of branched-chain ketoacids in urine can be advised.
- This enables mild to moderate cases of acute metabolic decompensation to be detected in a timely manner and managed at home.
- In these situations, knowledgeable healthcare professionals can assist with leucine limitation in the diet, sick day formulae, and outpatient monitoring
- Sick day guidelines ask for a 120% rise in the amount of BCAA-free amino acid formula consumed, 150 mL/kg of fluid administered, a 50%–100% reduction in leucine consumption, and frequent small feedings. Ensuring that the dietary therapy is vigorous and gives enough energy is crucial.
Within-Hospital Care
In-hospital therapy’s objectives and approaches include:
- managing the underlying stressor (such as fever, dehydration, infection, or inflammation) in an efficient manner.
- Antiemetics such as ondansetron should be used to control nausea and vomiting.
- a decrease in leucine levels of at least 750 micromol/L every 24 hours. Insulin and glucose infusions can be used to reduce leucine levels. Leucine levels should ideally be kept between 200 and 300 micromol/L. Total parenteral nutrition can be used to return 25–50% of the normal consumption of protein into the diet after clinical recovery. Over the next few days, this intake may be increased in accordance with the clinical circumstances.
- At least 1.25 times the weight or surface area must be supplied in EER. Between 40% to 50% of total calories should come from fat. Parenteral and enteral feeding can be used in tandem to meet nutritional objectives.
- One should supplement with 20–120 mg/kg/day of isoleucine and valine, respectively. Supplemental intake is modified to keep plasma concentrations between 400 and 600 micromol/L constant.
- Tyrosine supplementation by enteral (100–400 mg/kg/day) for the treatment of localized or widespread dystonias.
- supplementation of 150–400 mg/kg/day of alanine and glutamine.
- Boost with amino acids devoid of BCAAs.
- Keep your salt levels within the normal range.
- Address the underlying abnormalities of acid-base.
- Keep your daily osmolarity fluctuations under 5 mosm/L and keep your urine production steady.
- Treat and prevent hypophosphatemia and hypokalemia brought on by IV glucose and insulin therapy.
- Sodium Phenylbutyrate’s (NaPBA) function
- Sodium phenylbutyrate effectively scavenges nitrogen. It is mostly used to treat problems related to the urea cycle. Moreover, NaPBA can lower levels of branched-chain amino acids.
Other consideration
Orthotopic Liver Transplantation
Indication:
neuromotor impairments
inadequate regulation of metabolism
recurring metabolic adjustments
- Ten percent of BCKAD activity is expressed by the liver.
- Therefore, for patients with classic MSUD who are unmanageable, liver transplantation is advised.
- The liver of an unrelated, deceased person is used more frequently.
- Following transplantation, BCKAD residual activity may increase to levels comparable to mild MSUD.
- As a result, dietary limitations and periods of metabolic decompensation are typically less necessary after transplantation.
- It also doesn’t heal mental disorders, cognitive impairments, or brain damage that have already occurred.
- Nevertheless, transplantation does not stop cerebral edema from developing or stop more neurological deterioration from progressing.
Management in Pregnancy
- Yes, mothers with multiple personality disorder can give birth to a healthy child.
- The potential teratogenic consequences of high maternal leucine concentration should be explained to the mother. Tight metabolic control prior to and during pregnancy is essential in such patients.
- The need for BCAA and protein by the mother grows dramatically during the development of the fetus and placenta.
- Thus, in order to prevent potential nutritional deficits, measures of fetal development and plasma amino acid concentrations are essential.
- BCAA levels between 100 and 300 micromol/L are thought to be compatible with a typical baby’s birth.
- A referral to a metabolic center needs to be made at the time of delivery.
- This is due to the fact that the postpartum phase is thought to be hazardous for the mother.
- Metabolic decompensation can be caused by things like internal blood sequestration, labor stress, and uterine involution.
- Consequently, special precautions need to be taken to prevent catabolism in the postpartum phase.
Management of other MSUD Complications
Edema Cerebral
- Give out furosemide in doses of 0.5 to 1.0 mg/kg.
- 0.5–1.0 g/kg of mannitol infused, then 3%–5% of hypertonic saline
- Raising the head of the bed can help lower the chance of developing cerebral edema. In addition, PICC line usage is advised for patients with encephalopathy. Nutrition can be delivered with a PICC line without requiring an excessive amount of fluid.
Cerebral herniation
- Elevate the head.
- Use an endotracheal tube or face mask to induce hyperventilation.
- Add mannitol and a hypertonic saline solution.
Infection
- Patients with MSUD are more likely to develop fungal or bacterial infections on catheters as well as candida infections.
- Continuous monitoring of patients for hospital-acquired infections is essential.
- Acute metabolic decompensation may occur if they are not treated.
Acute Pancreatitis
- A patient may have mid-back pain, vomiting, anorexia, and epigastric pain during an acute metabolic decompensation episode.
- This should prompt the ordering of serum lipase and amylase levels and increase the possibility of acute pancreatitis.
- The patient must also be NPO and enteral feeding must be stopped by the doctor.
- The patient’s nutritional needs can be met with the use of specialized parenteral solutions, and treatment is typically supportive.
Neuropsychiatric Illnesses
- Patients who are adults or adolescents are more likely to have anxiety, depression, and ADHD.
- Medications such as psychostimulants and conventional antidepressants can be prescribed to treat them.
Secondary Complications
- Planning surgical operations and providing care for trauma patients should be coordinated with a metabolic specialist.
General prevention
- Patients need to be instructed not to take more branched-chain amino acids than is permitted each day.
Prognosis
- Patients who start therapy before or as soon as symptoms appear might expect a good prognosis.
- It is well established that plasma leucine concentrations influence neurocognitive results.
- In individuals of school age, classical MSUD has demonstrated superior performance to IQ.18] Of adult MSUD patients, 61% lead independent lives and successfully assimilate into society.
- Nonetheless, 56% of patients still need to receive psychiatric and psychological support.
- Developmental deficits may be mild in patients with late-onset MSUD, contingent on the level of residual BCKAD activity.
- Delays in diagnosing classic MSUD that extend beyond 7 to 14 days may cause cerebral palsy and lasting learning disabilities.
Difficulties
Serious repercussions may arise if MSUD is not diagnosed and treated in a timely way. Patients following a treatment plan may experience an acute sickness that causes branched-chain amino acid levels to spike unexpectedly. The onset of clinical symptoms such as excessive exhaustion, irritability, vomiting, and loss of awareness typically signals the onset of this metabolic crisis. The following consequences could occur if the patient is not diagnosed or treated:
- Seizures
- Metabolic acidosis
- Cerebral edema
- Cerebrovascular ischemia
- Intellectual disabilities
- Blindness
- Muscle spasticity
- Irreversible neurological damage
- Osteoporosis
- Acute pancreatitis
- Recurrence of candidiasis in the esophagus is caused by T-cell suppression due to elevated levels of plasma leucine. Essential amino acid deficiency presents as anemia, hair loss, growth failure, and acrodermatitis
Patient education and Deterrence
- Adherence to the best patient care strategies will be ensured by a full awareness of the clinical condition of the patient and the general public.
- Moreover, it enables doctors and other members of the healthcare team to deliver the greatest possible patient outcomes through the application of evidence-based medicine.
FAQ
Genes and Disease: Maple Syrup Urine Disease – NCBI Bookshelf
Urine that smells like maple syrup is one of the initial symptoms of an inherited condition known as Maple Syrup Urine Disease (MSUD). Certain amino acids are not metabolized properly due to the underlying problem.
Patients with MSUD who respond well to thiamine Because thiamine is a cofactor of the enzyme that causes MSUD, patients with the disorder may have increased protein intakes when taking high amounts of the vitamin. Depending on the level of enzyme activity, the usual daily dosage for thiamine-responsive MSUD might vary from 10 mg to 100 mg.
In the absence of the branched-chain ketoacid dehydrogenase (BCKAD) complex enzyme, the body becomes damaged by the accumulation of these amino acids and their metabolites. There are four broad categories of MSUD: thiamine-responsive, classic, intermediate, and intermittent. Among MSUD types, classic is the most severe.
The rare genetic condition known as maple syrup urine disease (MSUD) is typified by a lack of branched-chain alpha-keto acid dehydrogenase, an enzyme complex necessary for the body to metabolize the three branched-chain amino acids (BCAAs) valine, which isoleucine, and leucine.
One of the three genes—BCKDHA, BCKDHB, or DBT—mutates to produce maple syrup urine illness. These genes give the body the instructions it needs to produce BCKDH complex enzymes, which are necessary for the breakdown of amino acids like valine, isoleucine, and leucine.
References
- Professional, C. C. M. (n.d.). Maple syrup urine disease. Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/21168-maple-syrup-urine-disease
- Hassan, S. A. (2022, September 5). Maple syrup urine disease. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK557773