Emery-Dreifuss Muscular Dystrophy (EDMD)
Table of Contents
What is Emery-Dreifuss Muscular Dystrophy?
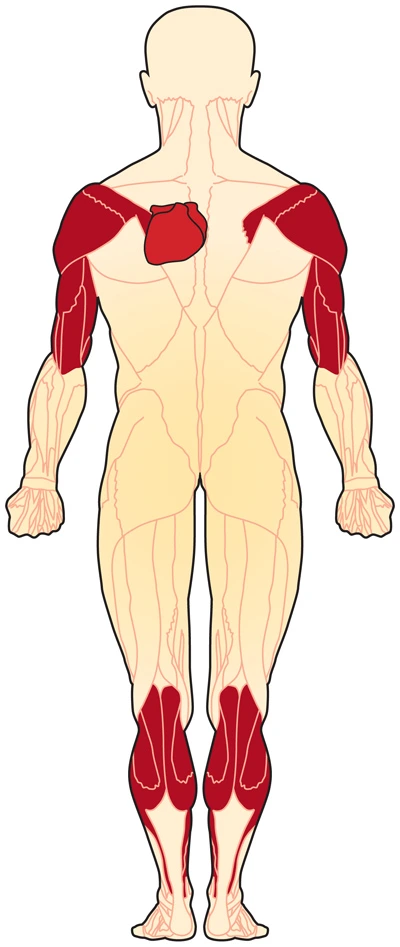
Emery-Dreifuss muscular dystrophy may be a condition that primarily affects muscles used for movement (skeletal muscles) and also the heart (cardiac muscle). Among the earliest features of this disease are joint abnormalities called contractures. Contractures restrain the movement of certain joints, most frequently the elbows, ankles, and neck, and frequently become conspicuous in infancy. Most affected individuals also encounter muscle weakness and wasting that aggravate slowly over time, beginning in muscles of the upper arms and lower legs and later also affecting muscles within the shoulders and hips.
Almost all people with Emery-Dreifuss hereditary condition develop heart problems by adulthood. In many cases, these heart problems are malformation of the electrical signals that control the heartbeat (cardiac conduction defects) and abnormal heart rhythms (arrhythmias). If untreated, these abnormalities can cause a sensation of fluttering or pounding within the chest (palpitations), a strangely slow heartbeat (bradycardia), fainting (syncope), heart disease, and an increased risk of extra time.
Researchers have determined several types of Emery-Dreifuss dystrophy that are distinguished by their pattern of inheritance: X-linked, autosomal dominant, and autosomal recessive. the categories usually have similar signs and symptoms, although any low percentage of individuals with the autosomal dominant form experience heart problems with no weakness or wasting of skeletal muscles.
Classification of Emery-Dreifuss Muscular Dystrophy
EDMD is one of the nine kinds of genetic disorders.
EDMD will be subdivided into 3 categories:
- X-linked EDMD
- Autosomal Dominant EDMD
- Autosomal Recessive EDMD
X-linked chromosome
The most common variety of EDMD is X-linked. this suggests that the changed gene is on the X chromosome. Chromosomes contain genes and are within the nucleus of your cells. Only boys get this manner of EDMD. Girls have two X chromosomes, from each parent. Boys get one X chromosome from their mother and a sex chromosome from their father. Because a boy only has 1 copy of the changed gene, a mutation means he won’t have enough of the protein to stay his muscles working well. a lady with a gene defect usually gets enough normal protein from her other sex chromosome. But she goes to be a carrier for the mutation and passes it on to her children.
Autosomal dominant
Another kind of EDMD affects both boys and girls, and either parent can pass this way all the way down to his or her children. The term for this type of inherited pattern is autosomal dominant. during this case, a parent with the disease incorporates a 1 in 2 chance of passing it on to every of his or her children.
Autosomal recessive
A rare kind of EDMD happens when a baby inherits a mutated gene from both parents (autosomal recessive). Both boys and girls may have this manner.
All 3 types of EDMD have similar symptoms. Researchers are still trying to know the connections between these defective proteins and also muscle weakness in EDMD.
Genetics
Mutations within one amongst several genes cause the varied styles of Emery–Dreifuss hereditary condition. Mutation of the EMD or LMNA gene is the cause in 40% of cases. Each gene implicated in EDMD provides instructions for creating a protein that’s related to the nuclear envelope, which surrounds the nucleus of a cell. The nuclear envelope regulates the movement of molecules into and out of the nucleus, and researchers believe it’s going to play a job in regulating the activity of certain genes.
Type:
EDMD1 – Emerin may be a transmembrane protein of the inner nuclear membrane which appears to be essential for the traditional function of skeletal and muscular tissue. Most EMD mutations prevent the assembly of any functional emerin.
EDMD2 – EDMD-causing mutations of LMNA end in the assembly of an altered version of their encoded proteins.
EDMD3 -EDMD-causing mutations of LMNA end in the assembly of an altered version of their encoded proteins.
EDMD4 – Muscle cells indicate loss of nuclear envelope consistency. Cerebellar ataxia occurs at approximately 30 years old.
EDMD5 – In SYNE2 we see a transition in the said gene, that leads to T89M as a result of substitution. Via fluorescent in-situ hybridization, the gene is found at chromosome 14q23.
EDMD6 -FHL1 is found at the sarcomere and sarcolemma, unlike the remainder of the proteins implicated in EDMD. it’s involved in sarcomere assembly.
EDMD7 – LUMA binds to emerin and LMNA and interacts with SUN2. it’s involved nuclear membrane structural organization and maintenance of shape, and deficiency of it causes abnormally shaped nuclei.
Characteristics/Clinical Presentation
EDMD is characterized by a triad of:
- Early Contractures (posterior cervical muscles, Achilles tendon, elbows, )
- Progressive atrophy of humeroperoneal distribution (proximal muscles of upper limb and distal muscles of lower limb).
- Late into the disorder, proximal limb girdle musculature becomes weak.
- Cardiac conduction defects.
Causes of Emery-Dreifuss muscular dystrophy- EDMD
Mutations in several genes, including EMD, FHL1, and LMNA, can cause Emery-Dreifuss hereditary disease. Mutations within the EMD gene or, less commonly, within the FHL1 gene cause the X-linked form of the condition. Mutations within the LMNA gene cause both the autosomal dominant and autosomal recessive varieties of the condition.
The genes related to Emery-Dreifuss dystrophy appear to be essential for the traditional function of skeletal and heart muscle. The EMD and LMNA genes provide instructions for creating proteins that are components of the nuclear envelope, which surrounds the nucleus in cells. The nuclear envelope regulates the movement of molecules into and out of the nucleus, and researchers believe it’s going to play a job in regulating the activity of certain genes. The protein produced from the FHL1 gene appears to be involved in other somatic cell functions, including chemical signaling, maintaining the structure of those cells, and influencing muscle growth and size.
Mutations within the EMD, FHL1 and LMNA genes that cause Emery-Dreifuss hereditary condition prevent the assembly of their respective proteins or cause abnormal or nonfunctional versions of those proteins. Researchers speculate that changes in EMD or LMNA could weaken the structure of the nuclear envelope in cells that undergo lots of mechanical stress, like skeletal and muscle cells, making these cells more fragile. FHL1 gene mutations also alter the structure and performance of muscle cells, although little is understood about the mechanism. Researchers still investigate how genetic changes can cause joint contractures, muscle weakness, and heart abnormalities characteristic of Emery-Dreifuss hereditary condition.
Changes in several other genes lead to conditions that resemble Emery-Dreifuss inherited disease, but with more variable features. Some researchers consider these to be forms of Emery-Dreifuss genetic disorder, while others believe that they represent similarly, but separate, disorders.
In more than 1/2 of all cases of Emery-Dreifuss congenital disease, the genetic reason for the condition is unknown. Researchers believe that mutations in additional genes, which haven’t been identified, may also cause this condition.
Inheritance:
Emery-Dreifuss dystrophy can have several different patterns of inheritance. When the condition is caused by mutations within the EMD or FHL1 gene, it’s inherited in an X-linked recessive pattern. A condition is taken into account X-linked if the mutated gene that causes the disorder is found on the sex chromosome, one in all the 2 sex chromosomes in each cell. In males (who have only 1 X chromosome), one altered copy of the gene in each cell is sufficient to cause the condition. Males are plagued by X-linked recessive disorders far more frequently than females. A feature of X-linked inheritance is that fathers cannot pass X-linked properties to their sons.
In females (who have two X chromosomes), a mutation typically must be present in both copies of the gene to cause X-linked Emery-Dreifuss congenital disease. Women who carry one changed copy of the gene usually don’t undergo the muscle weakness and wasting that are characteristic of this condition. However, many of these female mutation carriers develop heart problems or mild muscle weakness.
Although there’s a defining group of clinical findings, the proteins and underlying gene defects to blame for EDMD are different. Among the affected families, different inheritance patterns are observed. EDMD was first recognized as a unique kind of genetic disorder from DMD/BMD during a family that had an X-linked recessive inheritance pattern. Defects in EMD which encode nuclear envelope protein emerin were identified to be the reason for
disease (EDMD1).
Similarly, another inheritance sort of EDMD is autosomal dominant (AD-EDMD). Although AD-EDMD’s
prevalence is unknown, it appears to be more common compared to X-linked EDMD (X-EDMD).10) For autosomal dominant EDMD (EDMD2), a mutation in LMNA is the commonest cause. LMNA encodes lamins A and C, which are components of the nuclear envelope located within the lamina. Moreover, autosomal recessive inheritance of LMNA has been described (EDMD3).
Actually, additionally to EDMD, mutations in LMNA are related to various phenotypes, including limb-girdle dystrophy type 1B, autosomal recessive axonal neuropathy (CMT2B), and Hutchinson-Gilford progeria syndrome. Furthermore, for dilated cardiomyopathy (DCM), LMNA is listed collectively as the foremost common.
Pathogenesis
LMNA and EMD are the 2 most typical mutant genes that are identified in EDMD. Lamins A/C and emerin are the nuclear membrane proteins and components of a proteinaceous meshwork (the nuclear lamina), which plays a crucial role in maintaining the architecture and is a skeleton for various other nuclear factors involved in DNA reproduction, chromatin organization, and transcription. Muscle biopsies for both affected X-EDMD and AD-EDMD patients showed that myonuclei display an abnormal nuclear architecture, a breakdown of the fragile nuclear membrane, and chromatin reorganization.
In addition to specific localization at the inner nuclear membrane, in the heart, and in cultured rat cardiomyopathy, emerin was found to localize on the intercalated disc, which can account for characteristic conduction defects in EDMD. However, although mouse models are created and a number of other working hypotheses are developed, the pathogenic processes by which mutations in genes encoding nuclear envelope proteins cause muscle abnormalities in EDMD remain unclear.
Signs & Symptoms of Emery-Dreifuss Muscular Dystrophy:
The severity, age of onset, and progression of EDMD vary greatly from case to case, even among people of the same family. Some affected people may suffer childhood onset with rapid disease development and severe complications; others may experience adult onset and a slowly progressive course.
EDMD is related to clinical contractures, muscle weakness, and cardiomyopathy. A contracture occurs when the shortening and thickening of tissue cause abnormalities and restrict the movement of affected areas, especially the joints. The elbows and Achilles tendons are the foremost common sites for contractures. Contractures are often the primary sign-on X-linked EDMD and should occur early during childhood. In autosomal dominant EDMD contractures develop after the onset of muscle weakness.
Progressive muscle weakness and degeneration (atrophy) usually develop during late childhood or the early phase of adolescence usually within the upper arms and lower legs (humeroperoneal regions). Weakness and atrophy of leg muscles may cause affected children to run on their toes and should lead to an abnormal waddling gait. Muscle weakness affecting the arms may cause various problems like difficulty in raising the arms above the head.
Eventually, the muscles of the thigh and hips may get involved making it difficult to climb stairs. The neck, pectoral arch, and forearms may eventually get entangled and therefore the spine may become rigid. As affected individuals age, they will experience limited mobility of the neck. Weakness of facial muscles has also been announced. An abnormal arch of the spine (scoliosis) may additionally occur.
Muscle weakness and atrophy are sometimes slowly progressive during the primary three decades of life. Eventually, it should become more rapid. Some individuals with autosomal dominant EDMD may eventually lose the flexibility to steer (ambulate) and need a wheelchair. Loss of ambulation is rare in X-linked EDMD.
Heart abnormalities are the third prominent feature of EDMD and should end in serious complications. Although onset can differ, heart abnormalities usually develop after the second decade of life. Affected individuals may develop disease of the center muscles (cardiomyopathy) potentially leading to palpitations, fatigue, poor exercise tolerance, and an impaired ability of the guts to pump blood. Some individuals may experience conduction defects leading to irregular heartbeats (arrhythmias) or Adams-Stokes syndrome.
Heart block is characterized by interference with the transfer of the electrical nerve impulses (conduction) that regulate the traditional, rhythmic, pumping action of the guts muscle. the conventional heart has four chambers. the 2 upper chambers are the atria and therefore the two lower chambers are the ventricles. Within the proper atrium of a traditional heart may be a natural pacemaker that initiates and controls the heartbeat. The electrical stimulus travels from the pacemaker (sinoatrial or SA node) to the ventricles along a really specific path consisting of conducting tissue and is referred to as the AV (atrioventricular) node. As long as the electrical impulse is transmitted normally, the center behaves normally. If the transmission of the signal is impeded, the blocked transmission is understood as an atrioventricular block or an AV block.
Heart blocks are categorized in keeping with the degree of impairment. The severity of some conductivity abnormalities varies among individuals with EDMD. Within the mild style of Stokes-Adams syndrome, the 2 upper chambers of the center (atria) beat normally, but the contractions of the 2 lower chambers (ventricles) lag slightly behind. within the more severe forms, only a half to 1 / 4 of the atrial beats are conducted to the ventricles. In a complete atrioventricular block, the atria and ventricles beat separately. In some cases, Adams-Stokes syndrome may cause blackouts (syncope), breathlessness, and/or irregular heartbeats (arrhythmias). In severe cases, extra time is feasible.
Some other signs and symptoms are:
- Symmetric weakness of biceps and triceps with spare deltoids
- Face, thigh, and hand weakness is rare and occurs later within the disease
- Toe walking
- Cardiomyopathy (which may cause heart failure)
- Atrioventricular block
- Atrial paralysis
- Sudden death
- Fainting because of its effect on the heart
- Symptoms are often seen by age 10
- Cardiac abnormalities are usually detected by the age of 20
Women who are carriers of X-linked EDMD won’t have muscle involvement but may have cardiac abnormalities.
Children usually show symptoms of EDMD by 10 years old. you will first notice “toe-walking” or waddling. Common symptoms include:
- Weakness and wasting (atrophy) of muscles within the shoulders, upper arms, and calves
- Stiff joints make it hard to maneuver around. The joints are often within the elbow, neck, heel, and spine.
- Muscle weakness that slowly gets worse
- Fainting or fluttering heartbeat (palpitations) thanks to heart problems. These are usually seen by age 30.
- Muscle weakness in EDMD gets worse very slowly, and plenty of people are still able to walk late in life. Some people may ultimately need a wheelchair or any other help getting around.
Associated Co-morbidities
Individuals with EDMD present with a large type of cardiac abnormalities including atrial standstill, fibrillation, congestive coronary failure, and cardioembolic stroke. The population is at risk for the development of severe bradyarrhythmias which carries risk time, and supraventricular tachyarrhythmias which are related to a high risk for thromboembolic stroke. it’s important to notice that cardiac and muscular involvement hasn’t been shown to be closely related, with cases of severe cardiomyopathy occurring in patients with only mild muscular symptoms. It’s possible for stroke to be the primary clinical manifestation of EDMD.
Systemic Involvement
Emery Dreifuss inherited disease affects voluntary muscles, similarly because of the heart. Early symptoms include weakness and atrophy during a humeroperoneal distribution, that may eventually affect the scapular and pelvis muscles. Contractures will occur and might make arm, neck, ankle, and spine movements difficult, resulting in to “toe-walking” and difficulty bending the elbows.
Cardiac conduction deficits and arrhythmias may occur, leading to dilated cardiomyopathy, poor exercise tolerance, bradycardia, syncope, congestive cardiomyopathy, and an increased risk of stroke and extra time.
Women who are genetic carriers for X-linked EDMD may additionally be in danger of cardiac problems, with the chance increases with age. However, carriers tend to not present with muscle weakness or contractures.
Diagnosis of Emery-Dreifuss Muscular Dystrophy
Clinical Diagnosis The clinical diagnosis of EDMD is created supported by the presence of the triad listed within the Clinical Presentation section.
Other Nonspecific Clinical Findings:
Electromyograms (EMG) usually shows myopathic characteristics with normal nerve conduction studies. However, neuropathic patterns are detected in X-Linked EDMD and autosomal dominant EDMD.
CT scan shows a spread form of involvement in muscles including the biceps, soleus, peroneal, external vasti, gluteus, and paravertebral muscles. Findings within the calf and posterior thigh are reported in patients with Autosomal Dominant EDMD.
Nonspecific Laboratory Findings:
Serum CK Concentration could also be normal or moderately elevated up to 2-20x upper normal level. Increases are seen more often at the start of the disease as critical later stages.
Muscle Histopathology shows nonspecific myopathic or dystrophic changes: variation in fiber size, increase in internal nuclei, increase in endomysial animal tissue, and necrotic fibers. microscopy may show specific alterations in nuclear architecture. Muscle Biopsy is never performed for diagnostic purposes because of lack of specificity.
Immunodetection of Emerin (detected by immunofluorescence): 95% absent in people with X-Linked EDMD. Normally stated in people with Autosomal Dominant EDMD.
Immunodetection of FHL1 (detected by immunofluorescence). Absent or significantly reduced in people with FHL1-related X-Linked EDMD.
Genetic testing: It can determine the appearance of a particular fault that cause EDMD and may help to assume the course of the disease, in addition, it helps to assess the chance of passing the disease on to the following generation.
Differential Diagnosis
Emery Dreifuss’s genetic abnormality must be differentiated from other diagnoses, primarily, the opposite styles of muscular dystrophies.
Duchenne Muscular Dystrophy- First recognized at age 3 to six. Characterized by muscle weakness and atrophy starting within the pelvic area, attending to shoulders, and eventually most major muscles of the body.
Becker Muscular Dystrophy- It begins during the second or third decade of life. Affects males almost exclusively. Hip and shoulder muscles are weakened, and gait abnormalities develop, in addition to possible mild slowness.
Facioscapulohumeral hereditary condition (Landouzy-Dejerine Muscular Dystrophy)- Onset is typical during adolescence or early adulthood. It is characterized by weakness of the facial, shoulder, and upper arm muscles. Impaired ability to lift arms overhead, shut eyes and move the lips may occur. Eventually, weakness and atrophy may affect the lower extremity yet.
Limb-Girdle Muscular Dystrophy– a bunch of rare progressive genetic disorders characterized by atrophy and weakness of the hip and shoulder girdles.
Rigid Spine Syndrome- It is a rare neuromuscular disorder characterized by atrophy of muscles, hypotonia, weakness, and contractures. Scoliosis often occurs. May occur in conjunction with EDMD.
Myopathies- Diseases to contemplate may include dermatomyositis, polymyositis, and nemaline myopathy.
Myasthenia Gravis– an autoimmune disease of the peripheral nerves characterized by weakness with repetitive use of a muscle, followed by recovery during a period of rest. most ordinarily affects the bulbar muscles with fluctuating generalized weakness.
Spinal Muscular Atrophy- Presents with weakness and atrophy within the limbs, respiratory, and bulbar muscles.
Upper motor nerve fiber Clinical Signs:
- Weakness or paralysis
- Spasticity
- Increased tendon reflexes
- Babinski sign
- Loss of superficial abdominal reflexes
- Little, if any, muscle atrophy
Lower efferent neuron Clinical Signs:
- Weakness or paralysis
- Wasting and fasciculations of involved muscles
- Hypotonia or flaccidity
- Loss of tendon reflexes
- Normal abdominal reflexes and negative inborn reflex
- Neurological problems may additionally present with sensory deficits.
Treatment of Emery-Dreifuss Muscular Dystrophy:
There is no specific treatment for EDMD. Treatment is decided to support individual symptoms.
Genetic counseling is also needed for the individual and his/her family.
Medical Treatment
Medications can help when the center is impaired in this way.
Medications include:
- Antiarrhythmic drugs for AV disorder
- Anti-thromboembolic drugs to forestall cerebral thromboembolism of cardiac origin
Medications to avoid:
- Depolarizing muscle relaxants (i.e. succinylcholine)
- Volatile anesthetic drugs (i.e. halothane, isoflurane)
Pharmacologic and non-pharmacologic therapy for heart disease
ACE inhibitor
Following diagnosis, the extent of the disease should be established with regard to the center, the lungs, and also the muscles/bones (musculoskeletal). Metabolic functions should even be assessed, as lipodystrophy can go with EDMD, by measuring levels of sugar, cholesterol, and triglycerides within the blood. Tests that are useful for heart evaluation include echocardiography (echo), electrocardiography (EKG), cardiac MRI, and electrophysiology studies. The musculoskeletal function will be evaluated by a physiotherapist.
Nevertheless, the magnitude of disease and cardiac and respiratory complications should be controlled. EKG, Holter, and echo are recommended yearly, and extra tests are often indicated betting on heart involvement. Pulmonary function tests (PFTs) are suggested every 2–3 years, or yearly in those with respiratory involution.
Although the foundation reason behind EDMD isn’t currently treatable, its manifestations and complications are treated. Heart implications will be considered with medications (antiarrhythmics, beta-blockers, and ACE inhibitors), pacemakers, defibrillators, and sometimes heart transplantation.
Surgical Treatment
Release of contractures and treatment of scoliosis
A heart transplant is also needed for end-stage heart disease
Cardiac pacemaker or implantable cardioverter defibrillator for
AV block and conduction disorder
Orthopedic surgery
In some cases, orthopedic surgery could also be required to correct contractures but is sometimes not performed due to the high rate of contracture recurrence. inherited disorder News is strictly a news and knowledge website about the disease. It doesn’t provide medical advice, diagnosis, or treatment. Always seek the recommendation of your physician or other qualified healthcare providers with any questions you will have regarding a medical condition.
Physiotherapy Treatment
Affecting breathing will be addressed with therapy and, later in the disease, mechanical ventilation. Contractures and scoliosis will be addressed with orthopedic surgery. Mobility is often improved with therapy, physiotherapists, and mechanical aids (canes, orthoses, wheelchairs).
Contractures
Contractures develop early in Emery-Dreifuss genetic disorder (EDMD) and may worsen whether or not muscle strength doesn’t change. Preventing contractures is difficult, but maintaining range of motion with therapy may help to slow their development. Surgical release of contractures is challenging due to their tendency to recur.
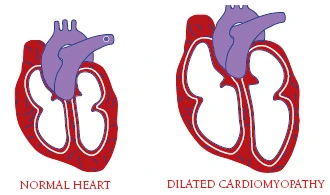
Cardiac conduction block
This type of heart disease occurs when the rhythm of the heartbeat is interrupted because the electrical impulses don’t communicate properly with the upper and lower chambers of the heart. Conduction block can cause sudden cardiopulmonary arrest. By age 30, the majority of those who have EDMD will have some type of noticeable cardiac involvement.
Cardiac problems may be life-threatening and should require the insertion of a pacemaker and treatment with medication. The issue is fairly easy to detect with an electrocardiogram. Anyone given a diagnosis of EDMD should be monitored routinely for signs of cardiac conduction block.
Cardiomyopathy
In addition to cardiac conduction abnormalities, many people with EDMD eventually develop dilated cardiomyopathy, damage to the heart muscle’s ability to pump blood around the body because it’s thinned out and floppy (dilated).
Occupational therapy
A trained occupational therapist can help patients to adapt their activities to complete the loss of muscle strength and stay independent while performing daily activities. they’ll recommend and train patients within the use of assistive devices like ankle and foot braces to forestall leg deformities, wheelchairs to assist with mobility, and home modifications like toilet bars, shower handles, dressing aids, and communication devices to facilitate interactive interaction.
Exercises
Active and Passive stretching to forestall the contracture
Active stretching: it’s a technique for improving flexibility. It involves the active contracting of 1 muscle (the agonist) as how to stretch an opposing muscle (the antagonist), with no external force.
Passive stretching: Passive stretching could be a technique within which you’re relaxed and make no contribution to the range of motion. Instead, an outdoor agent creates force, either manually or mechanically. Examples include employing a towel, band, gravity, or another person to assist you to stretch.
How does one reduce contractures?
Exercises to correct contractures — stretching exercises
Hold the limb in an exceedingly steady, stretched position while you count slowly to 25.
Then gradually stretch the joint a touch more, and again count slowly to 25.
Continue increasing the stretch during this way, steadily for five or 10 minutes. Repeat several times each day.
Nonsurgical options include:
- wearing open-back shoes, like clogs.
- taking NSAID drugs (NSAIDs), like ibuprofen (Advil, Motrin IB) or aspirin (Bufferin)
- icing the bump for 20 to 40 minutes per day to scale back swelling.
- getting ultrasound treatments.
- getting a soft tissue massage.
- wearing orthotics.
Range of motion exercises can help to scale back joint stiffness
Range of Motion Exercises: Range of motion exercise refers to activity geared toward improving the movement of a selected joint. This motion is influenced by several structures: configuration of bone surfaces within the joint, joint capsule, ligaments, tendons, and muscles engaged on the joint.
How does one reduce joint stiffness?
Hot or cold compress. Both temperature extremes could also be beneficial for stiff joints. Apply a chilly compress or bag of ice to your stiff joint for 15 to 20 minutes several times on a daily basis. this will help reduce inflammation or swelling and ease the joint into movement.
Active and passive exercises to assist in maintaining and building muscle strength
Examples of muscle-strengthening activities include:

Squats: Stand straight with feet hip-width apart. Tighten your stomach muscles. Lower down and try to sit as you sit on an invisible chair. Straighten your legs to lift and make a copy. Repeat the movement.
Bench press: Lie down on a bench on your back. Press your feet firmly into the bottom and keep your hips on the bench throughout the whole movement. Keep your core contact with the surface and maintain a neutral spine position throughout the movement. Slowly lift the bar or dumbbells off the pain if using.
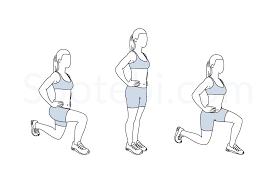
Lunge: Stand tall with feet hip-width apart. Engage your core. Take a giant discovery with your right leg. Lower your body until the right thigh is parallel to the ground and the right shin is vertical. Press into right heel to fight up to starting position. Repeat on the opposite side.

Burpees: Stand straight together with your feet shoulder-width apart. Squat and place your hands before your feet. Jump back, until your legs are fully extended and your body is in plank position. Do a push-up, jump forward, then come out the heels to return to the starting position.
Working with resistance band

1. Wall lateral pulldown: Stand together with your back against the wall. Place the resistor band around the thumbs or wrists and stretch the arms straight up over your head. Pull the arms down and the elbows are to the side, bent at a 90 degree of angle, during the stretching of the band and bringing your shoulder blades together and then Return to the starting position and repeat

2. Triceps extension: Hold the resistance band in your hands and keep along with the elbows bent. Put your right elbow over your head along with your right forearm parallel to the ground. The mitt should be before the left shoulder. Extend your right arm while keeping it near the pinnacle. because the right arm straightens, you ought to feel the band stretch and therefore the muscles of your right upper arm working. Return to the starting position.

3. Bicep curl: Sit on a chair, step, or on your heels. Tuck the resistance band underneath your right knee and hold it together with your paw. Pull the hand up, first towards the right side of the shoulder against the resistance of the band, and then your upper arm should stay immobile as you pull on the bar, keeping your elbow beneath your shoulder and shut to the body. After then, release the hold and back to the starting position. Do all repetitions on one side, then switch to the opposite side.

4. Shoulder external rotation: Place a band (mini) around your wrists and then bend your elbows and keep them near to your body. Move your forearms dead and set the side to stretch the band. Rotate your palms at the identical time, so they face once the band is stretched. Return to the starting position.
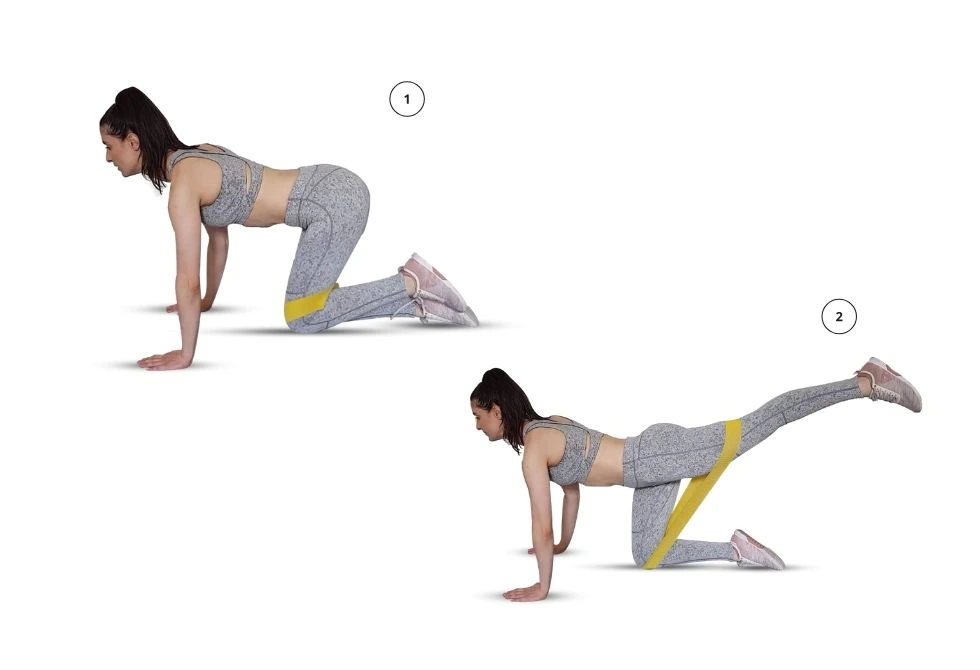
5. Donkey kick: Start on a card game. The resistance band should be above your knees. Keep your neck, back, and hips lined up. After then, Kick the left heel up to stretch the band. ensure you hold your hips level and don’t round your back. Return to the starting position. Do all repetitions on one side, then switch to the opposite side.

6. Squats: Begin standing together with your feet shoulder-width apart, toes parallel or slightly outward. The resistance band should be above your knees. Perform a squat so rise forcefully into a jump. Land softly on the balls of your feet. you’ll stand make a copy in between or continue with another jump.
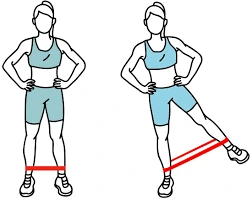
7. Hip abduction: Stand next to a wall and hold on thereto together with your hand for support. The resistance band should be placed around the ankles. After then, give resistance by moving the band above your knees. Lift the right leg to the side while squeezing your outer glute. Keep your body straight, don’t bend your torso. Return to the starting position. Do all repetitions on one side, then switch to the opposite side.
Complications of Emery-Dreifuss dystrophy
Heart problems are the foremost common complication in EDMD. the majority with the condition have some heart problems by age 30. People with EDMD must be closely monitored. Problems can include:
Irregular heartbeat.
This is caused by problems sending electrical signals from one part of the center to a different one. It can cause slowed heartbeat or missed heartbeats. Your child will need checkups a minimum of once a year for irregular heartbeats or other heart problems. this can be true whether or not your child has no symptoms. Your child might have a pacemaker or an implantable cardioverter-defibrillator (ICD) to cut back the chance of complete Stokes-Adams syndrome or sudden cardiopulmonary arrest.
Stretched and weakened muscular tissue (dilated cardiomyopathy).
This can make it hard for the center to pump enough blood through the body. Your child might have medicine like beta-blockers or angiotensin-converting enzyme (ACE) inhibitors.
Muscle weakness. This usually gets worse over time. it should spread to involve the hips and other muscles throughout the body. Weakness and wasting (atrophy) of muscles within the shoulders, upper arms, and calves. Stiff joints make it hard to maneuver around. The joints are often within the elbow, neck, heel, and spine.
Stiff joints that are difficult to maneuver (contractures). Your child may have aids like canes, braces, a walker, or a wheelchair.
Breathing problems. These are caused by weakened breathing muscles. A positive pressure respirator can give your child enough airflow.
Is Emery-Dreifuss fatal?
Emery-Dreifuss genetic abnormality (EDMD) may be a rare disease usually presenting in childhood with early contractures, slowly progressive humeral and posterior calf muscle weakness/atrophy, and potentially fatal dilated cardiomyopathy with conduction defects.
How common is Emery-Dreifuss Muscular Dystrophy?
The frequency of the general prevalence of Emery-Dreifuss hereditary disease is unknown. The X-linked sort of this disorder affects an estimated 1 in 100,000 people. The incidence of the autosomal dominant type is unknown, although it shows to be more common than the X-linked type.
What is the prognosis for Emery-Dreifuss Muscular Dystrophy?
Muscle weakness and atrophy are typically slowly progressive during the primary three decades of life. Eventually, it should become more rapid. Some individuals with autosomal dominant EDMD may eventually lose the flexibility to run (ambulate) and need a wheelchair. Loss of ambulation is rare in X-linked EDMD.
What organelle does Emery-Dreifuss’ inherited disease affect?
EDMD is caused by mutations in genes encrypting proteins of the nuclear covering, with definitive connections to mutations in EMD and LMNA, respectively, encoding emerin and A-type lamins and possible linkages to genes encoding other proteins of this nucleolus.
Is Emery-Dreifuss muscular dystrophy progressive?
Muscular dystrophies (MD) are a gaggle of inherited genetic conditions that gently cause the muscles to weaken, resulting in an increasing level of disability. MD could be a progressive condition, which implies it gets worse over time.
How is the nucleus affected Emery-Dreifuss muscular dystrophy?
The deficiency of some protein roles within the cell’s nucleus (emerin, lamin A, lamin C) ends up in Emery-Dreifuss dystrophy. a small protein called emerin, which normally is found within the membrane that surrounds each cell’s nucleus (the compartment in a very cell’s center that contains the chromosomes).