Neuropathic Pain
Table of Contents
What is Neuropathic Pain?
Neuropathic pain is a chronic pain condition caused by damage or disease to the somatosensory nervous system. This can include the peripheral nerves, spinal cord, and brain. Neuropathic pain can be difficult to treat, but there are a variety of medications and therapies that can help manage the pain and improve quality of life.
- Neuropathic pain may be experienced by your nervous system if it is damaged or not operating properly. Pain can originate from any of the several levels of the neurological system, including the brain, spinal cord, and peripheral nerves. The CNS includes the spinal column and brain. Your organs, arms, legs, fingers, and toes are only a few of the body’s peripheral nerves’ locations.
- Incorrect signals are sent to pain centers by damaged nerve fibers. Both the location of the damaged nerve and regions of the Nervous System (central sensitization) may experience changes in the way that the nerves operate.
- Neuropathy is a condition that affects the structure or function of one or more nerves. About 30% of instances of neuropathy are caused by diabetes. It is not always easy to find the source of neuropathic pain. Numerous illnesses are connected to this kind of pain.
What causes Neuropathic Pain, for instance?
- Alcoholism is one illness that can lead to neuropathic pain.
- Diabetes.
- Facial nerve issues.
- AIDS or HIV infection.
- diseases of the central nervous system (such as multiple sclerosis, Parkinson’s disease, and stroke) syndromes of complex regional pain.
- Shingles.
Other causes include:
- Chemotherapy drugs (cisplatin, paclitaxel, vincristine, etc.).
- Radiation therapy.
- Amputation, which can cause phantom pain.
- Spinal nerve compression or inflammation.
- Trauma or surgeries with resulting nerve damage.
- Nerve compression or infiltration by tumors.
Pathophysiology:
- A lesion in the peripheral or central nervous system, which can happen anywhere, can cause neuropathic pain.
- Other pain centres receive false signals from these injured nerve fibres.
- A change in nerve function at the injury site and in the surrounding regions is one effect of a damaged nerve fibre.
- Neuropathic symptoms can really start peripherally via ectopic impulses along the Aß, Ad, and C fibres. These ectopic impulses appear as spontaneous activity as a result of conditions such as accumulated broken ion channels along affected sensory axons, which results in a drift toward threshold potential.
- As the damaged nerve begins firing ectopically, A-fibre sprouting into the pain layers (laminae I and II of the dorsal horn) may occur. Pain may develop from non-noxious stimuli because of this sprouting process, which occurs when nerves that do not typically convey pain sprout into these more superficial laminas.
- Central sensitization may emerge from a peripheral origin as a result of ectopic neuronal activity in the dorsal horn of the spinal cord, suggesting a possible autonomous pain-generating mechanism. This might result in a rise in the size of the sensory receptive field, decreased pain threshold, and dorsal horn hypersensitivity to different harmless stimuli.
- The tissue inflammation can be sustained by the antidromic direction of the impulse produced in the AIGS. This might reduce the persistent chemical triggers causing peripheral nociceptive discomfort.
- It is still unclear what causes neuropathic pain. Numerous investigations on animals have revealed that there may be numerous systems at play.
- If first-order neurons are partly injured and develop more sodium channels, they may fire more often.
- Ectopic discharges, which cause both spontaneous pain and discomfort associated with movement, originate from increased depolarization at specific places in the fibre.
- At the level of the brain stem or dorsal horn (or both), inhibitory circuits may be damaged, enabling pain impulses to pass unchecked.
- Additionally, when someone has chronic pain or uses certain medicines, there may be changes in how their brain processes pain.
- Second- and third-order neurons become sensitive to pain and form a “memory” of it. Then, spinal neurons exhibit increased sensitivity and lower activation thresholds.
Symptoms:
Neuropathic pain can be described as a burning, shooting, tingling, or aching pain.
Most common symptoms are:
- Sensitivity to touch or temperature
- Numbness or weakness
- Muscle spasms
- Difficulty sleeping
- Depression and anxiety
- Neuralgic pain has characteristics that set it apart from other forms of pain, such as pain and sensory symptoms that persist beyond the healing process. Allodynia, which is the perception of non-noxious stimuli as painful, causalgia, and spontaneous pain are characteristic features in humans.
- Both “negative” symptoms (sensory loss and numbness) and “positive” symptoms (paraesthesias, spontaneous pain, enhanced pain sensibility) are present in neuropathic pain.
- ‘Pins and needles,” shooting, scorching, stabbing, and paroxysmal pain (electric shock-like) are examples of spontaneous pain. These sensations are frequently related to dysesthesias and paraesthesias. The patient’s sensory system, along with their overall health, emotions, concentration, and thinking, are all impacted by these encounters. The most frequent examples of delayed pain onset are central post-stroke pain (thalamic) and phantom limb pain. Both of these neuropathic pain conditions can develop months or even years after the initial injury.
- Mechanical spinal pain with radiculopathy or myelopathy, for example, is a common example of pain that combines neuropathic and nociceptive types.
Treatment of Neuropathic Pain
Treatment for neuropathic pain will vary depending on the underlying cause and the severity of the pain. There are a variety of medications and therapies that can be used to manage the pain, including:
- Pain relievers, such as ibuprofen or acetaminophen
- Prescription pain medications, such as opioids, anticonvulsants, or antidepressants
- Nerve blocks
- Spinal cord stimulators
- Physical therapy
- Occupational therapy
If you are experiencing neuropathic pain, it is important to see a doctor to get a diagnosis and discuss treatment options.
- Neuropathic pain frequently does not react well to conventional pain medications, and on occasion, it may get worse rather than better with time. Some people may have severe disabilities as a result of it. Since treating the underlying cause of neuropathic pain is frequently not possible, treating its symptoms is the main goal of care, which necessitates an interdisciplinary strategy that integrates therapies.
The interdisciplinary strategy incorporates:
- Medical supervision and the application of pharmaceuticals
- Physical therapy,
- general low-impact exercise,
- counselling,
- relaxation techniques,
- massage, and acupuncture;
Utilization of pharmaceutical therapy options and medical supervision:
- Outside of the usage of specialized pain management services, a number of pharmaceutical therapies can be utilized to control neuropathic pain. The manner in which therapy is started, the dosages employed, the sequence in which medications are introduced, whether therapeutic doses are reached, and if the proper sequencing of therapeutic classes is followed, all vary significantly.
- Another issue is that many commonly used drugs do not have the appropriate approval for the treatment of neuropathic pain, this may limit their use. There may be severe morbidity and ineffective pain management as a result of these factors.
commonly used pharmacological treatments :
- First-line therapy:
AEDs, or antiepileptic medicines.
- Gabapentinoids, 150–600 mg/day of gabapentin.
Adverse effects: fatigue, dizziness, swollen extremities, and impaired eyesight; - Pregabalin 300–3600 mg per day
Adverse effects: fatigue, dizziness, swollen extremities, and weight gain
TCAs, or tricyclic antidepressants :
10-150 mg/day of amitriptyline
AE urinary retention, QT prolongation (arrhythmia), suicide risk, and anticholinergic effects
SNRI, or Serotonin-norepinephrine reuptake inhibitors:
Duloxetine 20-120 mg/day
AE Nausea, dry mouth, constipation, ataxia, lethargy;
Venlafaxine 150–225 mg/day
AE Nausea, dizziness, fatigue, excessive sweating, and high blood pressure
- Secondary treatment:
Opioids:
25-400 mg/day of tramadol
AE: nausea/vomiting, diarrhea, fatigue, seizures, and ataxia;
50-600 mg/day of tapentadol
AE: Constipation, lethargy, convulsions, ataxia, nausea, and vomiting
Topical medicine Lidocaine 5% gel or patches
AE: Localised erythema, rash, and itching
patches 8% capsaicin
AE: Itching, erythema, and occasionally, elevated blood pressure
- Third-line therapy :
opioids that are strong; morphine 10–120 mg/day AE nausea, vomiting, diarrhea, lightheadedness, and sluggishness; 10-120 mg/day of oxycodone AE Constipation, lethargy, nausea/vomiting, and respiratory control
Botulinum toxin 25-300 U BTX-A 0.9% saline neurotoxin AE Pain from an injection
The safety of our patients might be affected by some drugs, so it’s vital for physiotherapists to be aware of this. Although medication isn’t exactly a physiotherapist’s job, being aware of its possible effects can be extremely helpful.
Interventional therapies, which administer drugs to targeted areas or alter certain brain structures, provide alternative therapeutic alternatives for some people with persistent neuropathic pain.
Several of the interventions include;
Spinal cord stimulation:
Involves applying a monophasic square-wave pulse at a frequency between 30 and 100 Hz, which causes the painful area to become numb.
Cortical stimulation:
It involves activating the pre-central motor cortex below the motor threshold using either invasive epidural or transcranial non-invasive techniques (such as repeated transcranial magnetic stimulation (TMS) and transcranial direct current stimulation).
Deep brain stimulation:
Potential deep brain stimulation sites for pain management include the internal capsule, different sensory thalamic nuclei, periaqueductal and periventricular grey, motor cortex, septum, nucleus accumbens, posterior hypothalamus, and anterior cingulate cortex.
Intrathecal treatments:
This provides a particular medication delivery alternative to the painful portion for those who have severe, chronic pain that is resistant to other forms of therapy.
Physical Therapy Treatment
- When medication alone is insufficient, physical therapy methods and rehabilitation approaches are crucial possibilities that must be taken into account. The physical aspect of the inflammation, stiffness, and soreness is treated physically with exercise, manipulation, and massage utilized in physical therapy, but it also encourages the body’s natural pain-relieving chemicals, which aid in the body’s self-healing process.
- Physical therapy works so well to relieve pain because of this two-pronged strategy.
- Exercise is helpful in reorganizing and sprouting the cortical body representation in addition to that; the significant alteration in the neuromatrix linked to chronic pain is a component of central sensitization. The usefulness of TENS treatment in treating painful peripheral neuropathy is supported by low-quality data. Otherwise, low-level laser treatment may have favourable benefits on the management of neuropathic pain analgesia.
- Neurostimulation methods including deep stimulation of the brain (DBS), spinal cord stimulation (SCS), CES ( cortical electrical stimulation ), and TMS ( transcranial magnetic stimulation ) have also been used to successfully treat neuropathic pain.
- It has been suggested that exercise and movement representation techniques, which incorporate therapies like motor imagery and mirror therapy and entail seeing and/or visualising normal pain-free motions, can be beneficial in the management of neuropathic pain.
- In recent years, mirror treatment has been utilized for patients suffering from strokes and complicated regional pain syndrome in addition to those with phantom limb pain. To support these treatments for neuropathic pain, there isn’t enough high-quality evidence, thus additional study is required.
Exercise:
- Your capacity to manage chronic pain will improve by exercising for just 30 minutes a day, at least three or four days a week. This will increase your muscular strength, endurance, joint stability, and flexibility. Keeping up a regular exercise routine will help with pain management.
- Regular therapeutic exercise will keep you physically fit and able to function, keeping you from losing your ability to move owing to your chronic pain.
- Studies suggest that exercise may have a significant role in both the prevention and management of neuropathic pain following chemotherapy. Although additional research is necessary and specific exercise recommendations for cancer patients are not currently available.
- Further research has shown that mechanical allodynia and heat hyperalgesia in animal models of neuropathic pain may be considerably reduced by physical exercises, such as swimming and forced treadmill running.
Balance Training
- Muscles and joints experience stiffness and weakness as a result of peripheral neuropathy. Building strength and easing tension are two benefits of balance training. Your risk of falling decreases as your balance improves. Calf and leg lifts are two examples of balance training exercises.
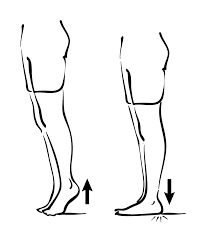
Calf Raise
- To maintain your equilibrium, Use a counter or chair.
- Stand on your toes while raising both heels off the floor.
- Slowly lower your weight.
- Repeat this exercise 15-20 times.
Side Leg Raise
- Using one hand, support yourself using a counter or chair.
- Stand straight with your feet apart.
- Slowly raise one leg to the side and maintain the posture for 5–10 seconds.
- Lower the leg gently.
- On the other leg, use the same method.
- With time, your posture will improve, so try the exercise without holding onto the counter.
Aerobic Exercise:
Your heart and respiratory rate rise during aerobic activity, which also tones your muscles. Aim for three to five sessions each week, each lasting 30 minutes.
Among the aerobic exercises are:
- brisk walking outside or on a treadmill
- doing aerobics sessions with a minimal impact
- Swimming
- Regularly cycling
Exercises for Flexibility or Stretching:
These exercises maintain joint flexibility and lower your risk of injury while participating in other activities. first, gently The body should be stretched to prepare for further stretching activities, such as the following:
Plantar fascia stretch:
- Face the door frame and position one heel as near to the edge as you can to do a plantar fascia stretch. Lean forward gradually, allowing your heel to revert as your toes curl upward. Until you feel a muscular stretch at the sole of your foot and the heel chord, bend the front knee towards the frame. Once you’ve held for 15 to 20 seconds, switch to the opposite leg.
Harmstring stretch in a seated position:
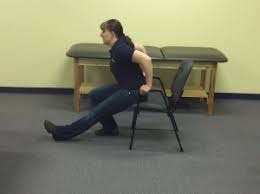
- Sit on the front of a chair and extend one leg with the toes pointed up to perform a hamstring stretch. Make sure the foot is on the ground and bend the opposing knee. Slowly straighten your back while moving your chest to the middle of the straight leg.
- The muscle at the rear of your leg should stretch. Alternate the legs for 20 seconds while holding.
- It is essential to use supportive shoes when working out to relieve neuropathic pain to avoid pressure and friction from socks. Use knee and elbow pads for protection, and don’t forget to remove layers of clothes to allow for good respiration.
FAQs
Persistent pain that results from damage or malfunctions to the nerve system is known as neuropathic pain. It frequently causes scorching, tingling, or shooting senses.
Neuropathic pain can result from conditions like diabetic neuropathy, postherpetic neuralgia (shingles), nerve injuries, and diseases like multiple sclerosis.
Diagnosis typically involves a detailed medical history, physical examination, and sometimes nerve conduction tests or imaging studies.
Treatment may include medications such as anticonvulsants, antidepressants, or opioids, physical therapy, nerve blocks, and lifestyle modifications.
Neuropathic pain is often chronic and may not be completely curable, but it can often be managed effectively with treatment.
Some people find relief through alternative therapies like acupuncture, massage, or biofeedback, but results can vary.
Maintaining a healthy lifestyle, managing underlying conditions (like diabetes), and avoiding alcohol and tobacco can help reduce the severity of neuropathic pain.
Untreated neuropathic pain can lead to decreased quality of life, emotional distress, and disability due to pain-related limitations.
Preventing the underlying causes of neuropathic pain, such as managing diabetes or avoiding injuries, can reduce the risk of developing it.
If you experience persistent or severe neuropathic pain, it’s important to consult a healthcare professional for proper diagnosis and treatment.


2 Comments