Metabolic Acidosis
Table of Contents
What is Metblic Acidosis?
Metabolic acidosis is a condition in which the body produces too much acid or when the kidneys are not removing enough acid from the body. This leads to a decrease in blood pH, which can cause a variety of symptoms and potentially life-threatening complications if left untreated.
Metabolic acidosis is an electrolyte disease described by an imbalance of acid-base balance in the body. 3 main root causes: increased acid production, a diminished ability of the kidneys to excrete excess acids, & loss of bicarbonate. It can cause acidemia, which is characterized as arterial blood pH that is lower than < 7.35. Acidemia & acidosis are not mutually exclusive pH & hydrogen ion attention also rely on the coexistence of other acid-base conditions: therefore, pH levels in people with metabolic acidosis can vary from low to high.
Acute metabolic acidosis: In this type of acidosis endures from minutes to many days, usually happens during intense illnesses or hospitalizations, & is generally generated when the body builds an extra amount of organic acids ketoacids in ketoacidosis or lactic acid in lactic acidosis.
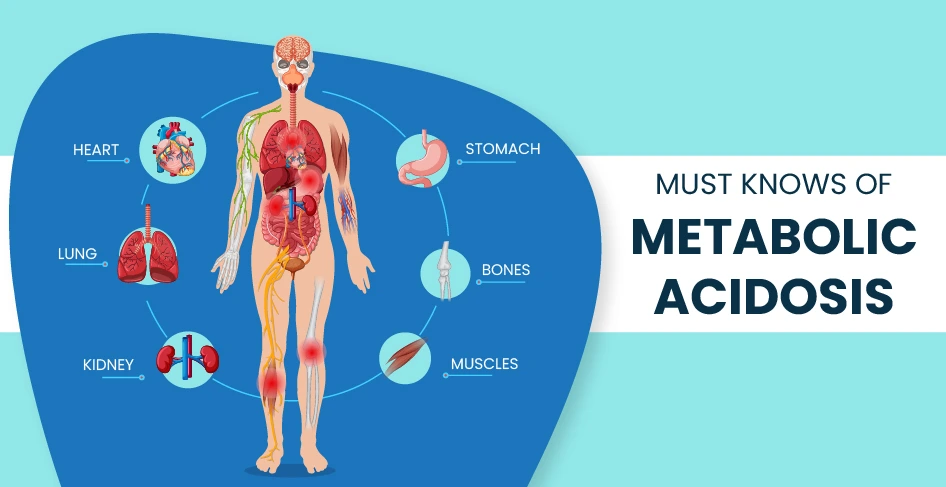
Chronic metabolic acidosis: In this type of acidosis, the development of impaired kidney function chronic kidney disease, & bicarbonate wasting. The damaging effects of acute versus chronic metabolic acidosis also differ, with acute metabolic acidosis affecting the cardiovascular system in the hospital, & chronic metabolic acidosis affecting kidneys, muscles, bones, & cardiovascular health.
What occurs to the body if have Metabolic Acidosis?
The body has a specific pH balance to function appropriately. The scale of pH levels of acids & bases in the blood. The pH scale ranges from very acidic to very basic or alkaline [ 0 to 14 ]. A normal pH range in the blood is from 7.35-7.45. The kidneys & lungs help keep a proper pH balance. The kidneys remove extra acids & bases from the blood via the urine. The amount of carbon dioxide in the blood is controlled by the lungs. When the body delivers too much acid, or the kidneys don’t release sufficient acids from the blood metabolic acidosis occurs.
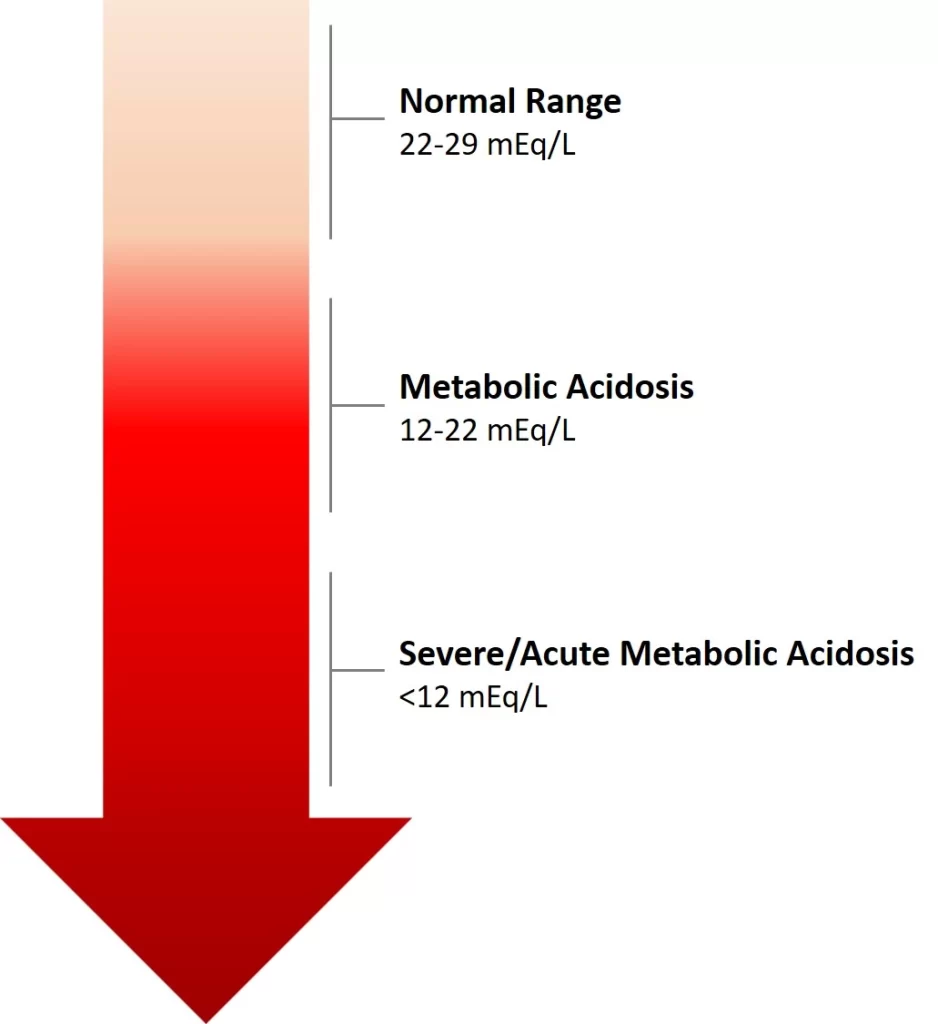
Causes of Metabolic Acidosis
The causes of metabolic acidosis can be classified into two main categories: increased acid production and decreased acid excretion. Increased acid production can occur due to a variety of factors, including uncontrolled diabetes, alcohol or drug abuse, and certain types of kidney disease. Decreased acid excretion can be caused by kidney failure or certain medications, such as acetazolamide or methotrexate.
Normally, metabolic acidosis happens when the body produces too much acid for example lactic acidosis. There is a defeat of bicarbonate from the blood, or when the kidneys are not discharging adequate acid from the body. Chronic metabolic acidosis is considerably caused by a decreased capacity of the kidneys to eliminate excess acids via renal ammonia genesis. The Western diet generates 75-100 mEq of acid daily, & people with normal kidney functions increase the production of ammonia to get rid of this dietary acid.
As kidney function decreases, the tubules lose the capability to excrete excess acid, & this results in buffering of acid using serum bicarbonate, as well as bone & muscle stores. There are many reasons for acute metabolic acidosis, & thus it is helpful to group them by the presence or absence of a routine anion gap.
Increased anion gap
Causes of increased anion gap contain:
- Lactic acidosis
- Ketoacidosis [ex: Diabetic, alcoholic, or starvation]
- Chronic kidney failure
- Transient 5-oxoprolinemia due to long-term ingestion of high doses of acetaminophen [usually seen with sepsis, liver failure, kidney failure, or malnutrition]
Intoxication:
- Salicylates, methanol, and ethylene glycol
- Organic acids, paraldehyde, ethanol, and formaldehyde
- Carbon monoxide, cyanide, ibuprofen, metformin
- Propylene glycol means metabolized to L and D-lactate & is usually discovered in infusions for certain intravenous medications utilized in the intensive care unit]
- Massive rhabdomyolysis
- Isoniazid, iron, phenelzine, valproic acid, tranylcypromine, verapamil
- Topiramate
- Sulfates
Normal anion gap
Causes of normal anion gap contain:
- Inorganic acid addition
- Infusion or ingestion of HCl, NH
- Gastrointestinal base loss
- Diarrhea
- Small bowel fistula or drainage
- Surgical diversion of urine into gut loops
Renal base loss or acid retention:
- Proximal renal tubular acidosis
- Distal renal tubular acidosis
- Hyperalimentation
- Addison disease
- Acetazolamide
- Spironolactone
- Saline infusion
To differentiate between the main types of metabolic acidosis, a clinical device called the anion gap is very helpful. The anion gap is estimated by subtracting the sum of the serum attention of major anions, chloride, & bicarbonate, from the serum concentration of the major cation, sodium. The serum potassium concentration may be counted in the calculation, but this only changes the normal reference range for what is believed a normal anion gap. Because the concentration of serum sodium is more significant than the combined concentrations of chloride & bicarbonate an ‘anion gap’ is mentioned. In reality, the serum is electroneutral because of the presence of other minor cations [ potassium, calcium & magnesium ] & anions [ albumin, sulfate & phosphate ] that are not counted in the equation that estimates the anion gap. The normal value for the anion gap is 8 to 16 mmol/L.
An elevated anion gap suggests the presence of extra ‘unmeasured’ anions, like lactic acid in anaerobic metabolism resulting from tissue hypoxia, glycolic & formic acid created by the metabolism of alcohols, ketoacids created when acetyl-CoA undergoes ketogenesis rather than joining the tricarboxylic cycle, & failure of renal excretion of products of metabolism such as sulfates and phosphates.
Adjunctive tests help determine the etiology of a raised anion gap metabolic acidosis including detection of an osmolar gap expressive of the presence of toxic alcohol, measurement of serum ketones expressive of ketoacidosis & renal function tests, and urinalysis to notice renal dysfunction. Elevated protein [ albumin, globulins ] may theoretically increase the anion gap but high levels are not generally undergone clinically. Hypoalbuminaemia, which is continually encountered clinically, will hide an anion gap. As a rule of thumb, a reduction in serum albumin by 1G/L will lower the anion gap by 0.25 mmol/L.
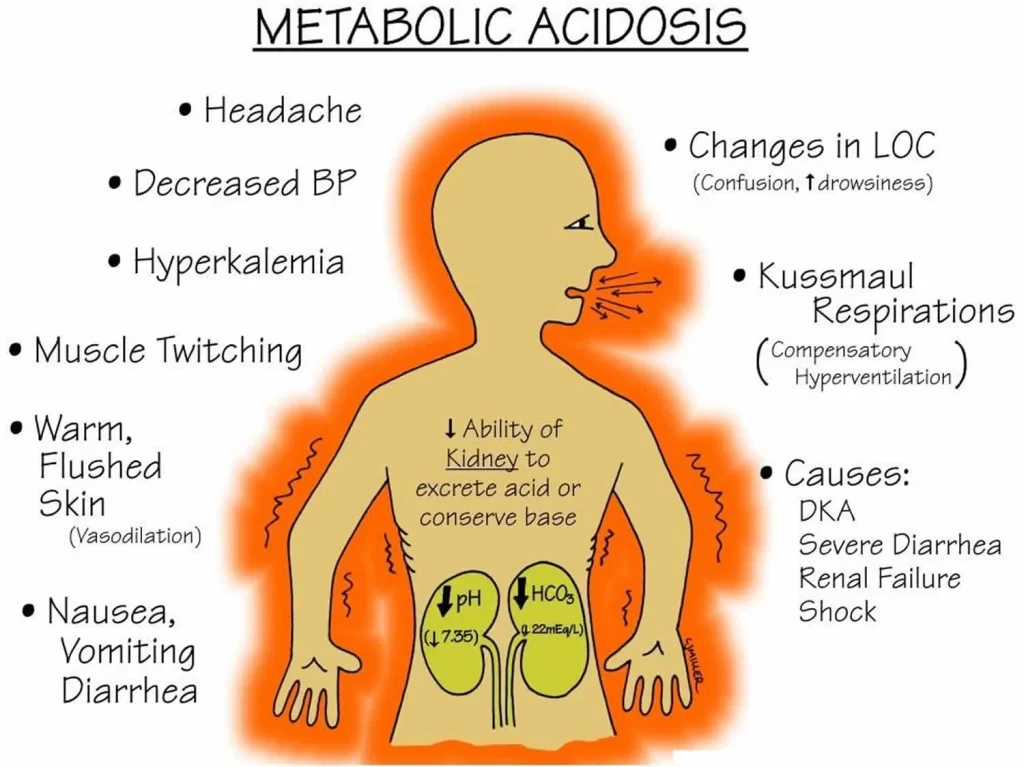
Signs and symptoms of Metabolic Acidosis
Symptoms of metabolic acidosis may include fatigue, shortness of breath, confusion, and a rapid heart rate.
Acute Metabolic Acidosis
Some symptoms are not distinctive, & diagnosis can be demanding unless patients present with clear signs for blood gas sampling.
Symptoms may contain:
- Palpitations
- Headache [altered mental status like extreme anxiety due to hypoxia]
- Reduced visual acuity
- Nausea
- Vomiting
- Abdominal pain
- Muscle weakness
- Bone pain
- Joint pain
- Fast deep breaths increase the amount of carbon dioxide exhaled, thus reducing the serum carbon dioxide levels, resulting in some degree of compensation.
Extreme acidemia can also cause neurological & cardiac complications:
Neurological: lethargy, stupor, coma, seizures
Cardiac: Abnormal heart rhythms [ e.g., ventricular tachycardia ] & decreased response to epinephrine, both coaching to low blood pressure
The physical examination can occasionally reveal symptoms of the disease but is often otherwise normal. Cranial nerve abnormalities are reported in ethylene glycol poisoning, & retinal edema can be a sign of methanol intoxication.
Chronic Metabolic Acidosis
Chronic metabolic acidosis has non-specific clinical signs but can be readily diagnosed by testing serum bicarbonate levels in patients with CKD [Chronic Kidney Disease] as part of a comprehensive metabolic panel. Patients with CKD Stages G3 to G5 should be routinely filtered for metabolic acidosis.
Common signs and symptoms of metabolic acidosis contain:
- Tachycardia (accelerated heartbeat)
- Confusion or dizziness
- Fatigue (Feeling very tired)
- Loss of appetite
- Headache
- Rapid breathing or long, deep breathing.
- Nausea & vomiting
- Feeling weak
- Breath that smells sweet or fruity
Diagnosis of Metabolic Acidosis
The healthcare provider will ask about the symptoms & may conduct a physical examination. They’ll also request tests to help ensure their diagnosis. They may also direct the to a nephrologist. A nephrologist is a physician who specializes in kidney disorders.
What test will be done for the diagnosis of Metabolic Acidosis?
The physician provider orders various tests according to what they acknowledge may be the reason for metabolic acidosis. These tests may include:
Blood tests: These tests help provide essential information about chemicals in the blood. These tests for metabolic acidosis may include:
Anion gap: The healthcare provider will use a thin needle to remove a small amount of blood from a vein in the arm. They’ll compare the distinction between the blood’s positively charged electrolytes & negatively charged electrolytes. A large gap between the blood’s positive & negative electrolytes may show metabolic acidosis.
Arterial blood gas (ABG): Healthcare will use a thin needle to remove a small amount of blood from an artery in the wrist, arm, or groin. Calculate the oxygen & carbon dioxide levels in the blood. Also, measure the blood’s pH balance. A blood pH below 7.35 suggests that they have too much acid or too little base in the blood.
Urine tests: During this test, collect urine into a special cup. The healthcare provider will check the pH level of the pee. May have too many acids in the pee or not enough bases in the pee.
Treatment for Metabolic Acidosis
Treatment depends on the underlying cause of the condition and may involve addressing the underlying medical issue, administering intravenous fluids and medications to restore acid-base balance, or even dialysis in severe cases. When regarding a course of treatment, it is necessary to distinguish between acute versus chronic conditions.
Bicarbonate: All need bicarbonate a form of carbon dioxide in the blood. Low bicarbonate levels in the blood are a symptom of metabolic acidosis. It is an alkali also known as base, the contrary of acid, and can balance the acid. Healthy kidneys help keep the bicarbonate levels in balance. Low bicarbonate levels of less than 22 mmol/l can also cause kidney disease to get worse. A group of investigations have shown that treatment with sodium bicarbonate or sodium citrate pills can assist keep kidney diseases from getting worse. However, should not take sodium bicarbonate or sodium citrate pills unless the healthcare provider recommends it.
Diet: Increasing fruit & vegetable intake may decrease the acid load in the body. Fruits & vegetables produce alkalis. The foods like meats, eggs, cheese, & cereal seeds can cause the body to make acids. The kidney dietitian can show you how to safely increase the right type & amounts of fruits & vegetables in the diet based on the stage of kidney disease.
Acute Metabolic Acidosis
Bicarbonate treatment is normally assisted in patients with severe acute acidemia i.e. pH < 7.11, or with less severe acidemia who have severe acute kidney injury. Bicarbonate treatment is not recommended for people with less extreme acidosis (pH ≥ 7.1) unless severe acute kidney harm is present. In the BICAR-ICU trial, bicarbonate therapy for maintaining a pH >7.3 had no overall impact on the composite outcome of all-cause mortality & the presence of at least one organ failure at day 7. However, amongst the sub-group of patients with painful acute kidney impairment, bicarbonate treatment greatly diminished the primary composite development, & 28-day mortality, along with the need for dialysis.
Chronic Metabolic Acidosis
For people with chronic kidney disorders, treating metabolic acidosis delays the progression of chronic kidney disorders. Recent research has also indicated that dietary protein restriction, through ketoanalogue-supplemented vegetarian very low protein diets, is also a nutritionally safe choice for correction of metabolic acidosis in people with a chronic kidney disorder. Currently, the most generally used treatment is oral bicarbonate. The NKF or KDOQI guidelines suggest starting treatment when serum bicarbonate levels are <22 mEq/L, to maintain levels ≥ 22 mEq/L. Investigations investigating the effects of oral alkali therapy demonstrated improvements in serum bicarbonate levels, resulting in a slower decline in kidney function, & a reduction in proteinuria – directing to a reduction in the risk of progressing to kidney failure. However, side results of oral alkali therapy contain gastrointestinal intolerance, worsening edema, & worsening hypertension. Furthermore, large doses of oral alkali are required to treat chronic metabolic acidosis, & the pill load can limit adherence.
Veverimer is a promising investigational drug designed to treat metabolic acidosis by binding with the acid in the gastrointestinal tract & removing it from the body via excretion in the feces, in turn decreasing the amount of acid in the body, & increasing the level of bicarbonate in the blood. Results from a Phase 3, double-blind placebo-controlled 12-week clinical trial in people with CKD & metabolic acidosis revealed that Veverimer effectively & safely corrected metabolic acidosis in the short-term, & a blinded, placebo-controlled, 40-week extension of the trial assessing long-term safety, demonstrated sustained improvements in physical function and a connected endpoint of death, dialysis, or 50% decrease in eGFR.
What should eat or drink if have Metabolic Acidosis?
Specific foods & drinks can lead the body to make more acids. Before making any differences to the diet, talk to the healthcare provider. They can advise on safely incorporating or increasing the right foods or drinks in the diet. They may also guide a dietitian who specializes in kidney disorders which means renal dietitian.
Foods & drinks that cause the body to make acids contain:
- Meats, including poultry and fish
- Eggs
- Cheese
- Grains
- Alcohol
Foods or drinks that deliver alkali contain:
- Fruits
- Nuts
- Legumes
- Vegetables
- Alkaline water
Consequences
Acute Metabolic Acidosis
Acute metabolic acidosis most frequently occurs during hospitalizations & acute critical illnesses. It is usually associated with poor prognosis, with a mortality rate as high as 57% if the pH stays untreated at 7.20. Lower pH levels and acute metabolic acidosis can generate impaired circulation & end-organ techniques.
Chronic Metabolic Acidosis
It is mostly seen in people with chronic kidney disease with an eGFR of less than 45 ml/min/1.73m2, with mild to moderate severity: although, metabolic acidosis can be shown earlier on in the study of chronic kidney disease. Many analyses have found that metabolic acidosis in chronic kidney disorder, due to its chronic nature, also has a negative effect on cellular function, overall donating to high morbidities in patients.
The most adverse effects of chronic metabolic acidosis in people with chronic kidney disease & in particular, for those who have end-stage renal disease (ESRD), are detrimental changes to the bones and muscles. Acid buffering cause loss of bone density, resulting in an increased risk of bone fractures, renal osteodystrophy, & bone disease as well, as improved protein catabolism causes muscle wasting. Furthermore, metabolic acidosis in chronic kidney disease is also associated with a reduction in eGFR it is both a difficulty of chronic kidney disease, as well as an underlying cause of chronic kidney disease advancement.
Risk factors for Metabolic Acidosis
Aspects that can contribute to the risk of metabolic acidosis contain:
- a high-fat diet that’s low in carbohydrates
- kidney failure
- obesity
- dehydration
- aspirin or methanol poisoning
- diabetes
- carbon monoxide poisoning
Complications of Metabolic Acidosis
Osteoporosis [Increased bone loss]: Metabolic acidosis can cause a loss of bone in the body. This can cause a higher chance of fractures in important bones like the hips or backbone.
Progression of kidney disorder: Metabolic acidosis can make the kidney condition worse. Precisely how this occurs is not clear. As acid builds up, kidney function lowers & as kidney procedure lowers, acid builds up. This can cause the progression of kidney disorders.
Muscle loss: Albumin is an essential protein in your body that helps build & keep muscles healthy. Metabolic acidosis lowers the amount of albumin created in the body, and causes muscle loss, or what is called “muscle wasting.”
Endocrine disorders: Metabolic acidosis inhibits the body’s ability to maintain normal functions of the endocrine system which means the collection of glands that produce hormones. This can cause the body to build insulin resistance the hormone in your body that helps keep the blood sugar level from getting too high or too low. If left untreated for too long or not repaired in time, it can cause diabetes.
Prevention
Can’t prevent metabolic acidosis. However, can help reduce the risk by:
- Drinking plenty of water & other fluids.
- Managing the blood sugar levels if have diabetes.
- Reducing the amount of alcohol that consume. Moderate alcohol consumption in men & people assigned male at birth is two drinks or fewer per day. In women & people assigned female at birth, moderate alcohol consumption is one drink or fewer per day.
Prognosis
- If have metabolic acidosis, the outlook depends on its harshness & what’s causing the situation.
- Many cases of metabolic acidosis react well to treatment after a proper diagnosis.
- In mild cases, the symptoms may be temporary, & may not require treatment.
- Many cases may include kidney or any other organ failure & death.
How do you take care of yourself?
The healthcare provider develops a treatment plan, which may contain medications & changes to the lifestyle.
The treatment plan may contain:
- Monitoring the blood sugar closely.
- Taking drugs as defined by the healthcare provider.
- Eating low-acid, high-alkali foods.
- Limiting the amount of alcohol consumed.
When should you contact Doctor?
Contact the healthcare provider if have any symptoms of metabolic acidosis or symptoms of a condition that may lead to metabolic acidosis.
FAQ
Low blood sugar hypoglycemia Medicines, like salicylates, metformin, & antiretrovirals. Melas is a very rare genetic mitochondrial disease that impacts energy production. Prolonged absence of oxygen from shock, heart defeat, or severe anemia.
Low bicarbonate levels in the blood are a symptom of metabolic acidosis. It is an alkali also known as base, the negative of acid, & can balance acid. It keeps our blood from evolving too acidic. Healthy kidneys help maintain the bicarbonate levels in balance.
Diabetic acidosis also called diabetic ketoacidosis & DKA forms when substances called ketone bodies which are acidic build up during unchecked diabetes. Hyperchloremic acidosis is led to the loss of too much sodium bicarbonate from the body, which can occur with extreme diarrhea.
IV sodium bicarbonate
Adding a base to counter high acid levels treats some types of metabolic acidosis. Intravenous treatment with a base called sodium bicarbonate is one way to balance acids in the blood. It’s used to treat conditions that cause acidosis via bicarbonate base failure.
In patients in whom volume depletion is attended by metabolic acidosis, sodium bicarbonate rather than sodium chloride may be the desired solution. This strategy may be particularly useful in patients who lost bicarbonate due to gastrointestinal losses.
Common electrolyte imbalances contain hyponatremia, hypokalemia, hyperkalemia, hypocalcemia, hypochloremia, & hypophosphatemia. Acid-base imbalances, either acidemia or alkalemia, occur as a result of the addition of acid & depletion of alkali reserve, or the loss of acid with a relative growth in alkali reserve.
The alkaline electrolyte is a concentrated aqueous solution of KOH, NaOH, & LiOH, 5–9 M. Relying on the application, the composition is the result of a compromise of the high-rate, low-, & high-temperature, & cycle life conditions.
spring water
Without a doubt, spring water is the winner. It is considered the best water to drink, providing vital nutrients as it transfers via the body. This is, of course, spring water that is bottled at the source & proven to be true residence spring water.