Disease Modifying Anti-rheumatic Drugs
Table of Contents
Introduction
Disease-modifying antirheumatic drugs (DMARDs) are a class of medications used primarily in the treatment of autoimmune diseases, particularly rheumatoid arthritis (RA) and other forms of inflammatory arthritis.
These drugs work by modifying the course of the disease rather than just alleviating symptoms. The introduction of DMARDs has significantly transformed the management of rheumatic diseases, providing patients with the potential for improved long-term outcomes.
Rheumatic diseases, including RA, psoriatic arthritis, and ankylosing spondylitis, are characterized by inflammation and damage to joints and other tissues. DMARDs play a crucial role in managing these conditions by suppressing the underlying immune response responsible for the inflammation.
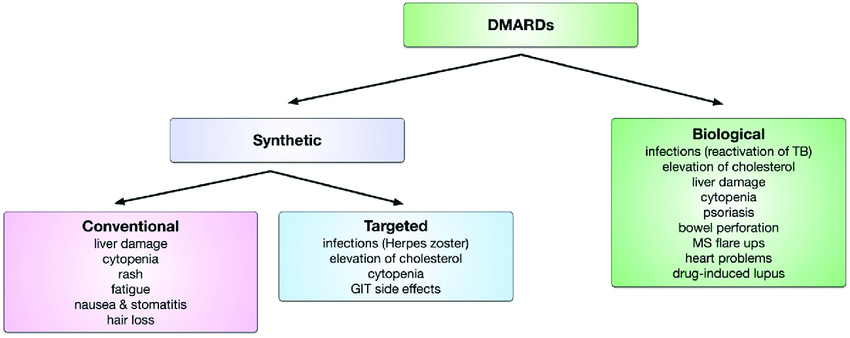
Types of disease-modifying antirheumatic drugs
There are two types of DMARDs,
- Conventional Synthetic DMARDs (csDMARDs): These drugs have been used for several decades and include medications such as methotrexate, hydroxychloroquine, sulfasalazine, and leflunomide. They act on various aspects of the immune system, reducing inflammation and preventing joint damage.
- Biologic DMARDs (bDMARDs): These are a newer class of drugs that are typically proteins produced using biotechnology. Specific immune system components are targeted by biologics, including
Tumor necrosis factor (TNF) inhibitors: Examples include etanercept (Enbrel®), infliximab (Remicade®), and adalimumab (Humira®).
Interleukin-1 inhibitor: An example is anakinra (Kineret®).
Interleukin-6 inhibitors: Tocilizumab (Actermra®) and sarilumab (Kevzara®).
T-cell inhibitor: An example is abatacept (Orencia®).
B-cell inhibitor: An example is rituximab (Rituxan®).
Janus kinase inhibitors: Biosimilars is another term for these drugs. Tofacitinib (Xeljanz®), baricitinib (Olumiant®), and upadacitinib (Rinvoq®) are a few examples.
The choice of DMARD depends on factors such as the specific autoimmune disease, its severity, and the patient’s response to different medications.
It is important to note that DMARDs are typically prescribed and managed by rheumatologists, specialists who focus on the treatment of autoimmune and inflammatory conditions. Regular monitoring is essential to assess the effectiveness of the treatment, manage potential side effects, and make adjustments to the therapeutic plan as needed.
Pharmacological effects of disease-modifying antirheumatic drugs:
Disease-modifying antirheumatic drugs (DMARDs) exert their pharmacological effects by targeting various components of the immune system and inflammatory pathways. The specific mechanisms of action vary among different DMARDs, but their overall goal is to modify the course of autoimmune diseases, particularly rheumatoid arthritis (RA) and other inflammatory forms of arthritis. Here are some general pharmacological effects of DMARDs:
Suppression of Inflammation:
DMARDs, both conventional synthetic DMARDs (csDMARDs) and biologic DMARDs (bDMARDs), work to dampen the inflammatory response that is characteristic of autoimmune diseases. By suppressing inflammation, these drugs help alleviate symptoms and prevent further damage to joints and tissues.
Immunomodulation:
csDMARDs, such as methotrexate, act by modulating the immune system. Methotrexate, for example, inhibits the activity of an enzyme involved in cell division, affecting rapidly dividing cells, including immune cells. This modulation helps regulate the abnormal immune response seen in autoimmune diseases.
Targeted Inhibition of Specific Molecules:
bDMARDs, which include drugs like TNF inhibitors (e.g., adalimumab, etanercept), interleukin inhibitors (e.g., tocilizumab, anakinra), and B-cell depleting agents (e.g., rituximab), target specific molecules or cells involved in the inflammatory process. By inhibiting these targets, they interrupt the signaling pathways that contribute to disease progression.
Reduction of Joint Damage:
One of the primary goals of DMARD therapy is to prevent or slow down joint damage. By controlling inflammation and modifying the immune response, DMARDs help protect joints from the erosive effects associated with autoimmune diseases.
Improved Function and Quality of Life:
By addressing the underlying cause of autoimmune diseases, DMARDs can lead to improvements in physical function, reduce pain and stiffness, and enhance the overall quality of life for individuals with these conditions.
Prevention of Systemic Complications:
In addition to their effects on joints, DMARDs may also help prevent systemic complications associated with autoimmune diseases. For example, certain DMARDs can reduce the risk of cardiovascular events that are elevated in some autoimmune conditions.
Why do we use disease-modifying antirheumatic drugs?
Disease-modifying antirheumatic drugs (DMARDs) are used in the treatment of autoimmune rheumatic diseases, particularly conditions like rheumatoid arthritis (RA) and other inflammatory forms of arthritis. The primary objectives of using DMARDs include:
Modify Disease Progression:
The main goal of DMARD therapy is to modify the course of the autoimmune disease. Unlike symptomatic relief provided by painkillers or anti-inflammatory drugs, DMARDs aim to slow down or halt the progression of the disease itself.
Prevent Joint Damage:
Autoimmune diseases like RA are characterized by chronic inflammation that can lead to joint damage and deformities over time. DMARDs help prevent or minimize this damage, preserving joint function and improving long-term outcomes.
Control Symptoms:
DMARDs are effective in controlling the symptoms associated with autoimmune diseases. This includes reducing joint pain, swelling, and morning stiffness. By addressing the underlying cause of inflammation, DMARDs provide more comprehensive symptom relief compared to medications that primarily target pain or inflammation.
Improve Physical Function:
By suppressing the inflammatory response, DMARDs contribute to improved physical function and mobility. This is crucial for maintaining a good quality of life and preventing disability associated with chronic autoimmune diseases.
Enhance Quality of Life:
A person’s quality of life can be greatly impacted by autoimmune illnesses. DMARDs, by effectively managing the disease, help patients lead more normal, active lives with less pain and disability.
Reduce Systemic Complications:
Some autoimmune diseases, such as RA, can have systemic effects beyond the joints, affecting organs like the heart, lungs, and blood vessels. DMARDs may help reduce the risk of these systemic complications.
Minimize the Need for Steroids:
While corticosteroids are sometimes used to manage acute symptoms, long-term use can lead to side effects. DMARDs are often used to reduce the reliance on steroids, helping to manage the disease with a more targeted and balanced approach.
Rheumatologists, who specialize in the treatment of autoimmune and inflammatory conditions, typically determine the most appropriate DMARD regimen for each patient based on a thorough assessment of their condition. Regular monitoring is essential to assess the effectiveness of the treatment and manage any potential side effects.
Who can take disease-modifying antirheumatic drugs?
Disease-modifying antirheumatic drugs (DMARDs) are typically prescribed for individuals diagnosed with autoimmune rheumatic diseases, especially those with conditions like rheumatoid arthritis (RA), psoriatic arthritis, ankylosing spondylitis, and others.
Diagnosis of Autoimmune Rheumatic Disease:
DMARDs are primarily prescribed for individuals diagnosed with autoimmune rheumatic diseases, where the immune system mistakenly attacks the body’s own tissues, especially the joints. Common indications include rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
Disease Severity:
The decision to initiate DMARD therapy often depends on the severity of the autoimmune disease. In cases where the disease is mild or in the early stages, healthcare providers may initially prescribe nonsteroidal anti-inflammatory drugs (NSAIDs) or corticosteroids. DMARDs are usually considered when the disease is more severe or not well-controlled with other medications.
Potential for Joint Damage:
DMARDs are often recommended when there is a risk of joint damage or deformity associated with the autoimmune disease. They are especially crucial for preventing long-term structural damage to joints in conditions like rheumatoid arthritis.
Persistent Symptoms:
If individuals experience persistent symptoms such as joint pain, swelling, morning stiffness, and fatigue despite other treatments, DMARDs may be considered to address the underlying inflammation.
Early Intervention:
In some cases, rheumatologists may opt for early intervention with DMARDs, especially in diseases like rheumatoid arthritis, where early treatment has been shown to improve outcomes and increase the likelihood of achieving remission.
Individual Health Factors:
The decision to prescribe DMARDs is also influenced by individual health factors, such as comorbidities, overall health status, and potential contraindications to specific medications.
It’s important to note that DMARDs are powerful medications that require careful monitoring by healthcare professionals. The choice of a specific DMARD and the treatment approach is highly individualized and depends on the patient’s unique circumstances. Regular follow-up appointments and monitoring for potential side effects are essential aspects of DMARD therapy. If you suspect you may benefit from DMARDs or have concerns about your treatment, it’s crucial to discuss these with your rheumatologist or healthcare provider.
Contraindication of disease-modifying antirheumatic drugs:
There are certain contraindications—factors or conditions that make the use of these medications inadvisable or potentially risky. Contraindications may vary depending on the specific DMARD. Generally speaking, the following should be taken into account:
Infections:
Many DMARDs, particularly biological DMARDs, and some conventional synthetic DMARDs, can suppress the immune system. Therefore, their use may be contraindicated in individuals with active infections or a history of recurrent, severe infections.
Pre-existing Liver Disease:
Some DMARDs, such as methotrexate, can affect the liver. Therefore, individuals with pre-existing liver diseases may have contraindications to certain DMARDs.
Blood Disorders:
Certain DMARDs, including methotrexate, can affect the bone marrow and blood cell production. Individuals with pre-existing blood disorders or conditions that affect the bone marrow may have contraindications to specific DMARDs.
Allergic Reactions:
Individuals with known allergies or hypersensitivity to specific DMARDs or their components may have contraindications to those drugs.
Heart Failure:
Some DMARDs, especially certain biologic agents, may have contraindications or require caution in individuals with heart failure or compromised cardiac function.
Pregnancy and Breastfeeding:
The use of certain DMARDs may be contraindicated during pregnancy or breastfeeding due to potential risks to the fetus or newborn. It’s important to discuss family planning considerations with healthcare providers.
Other Medication Interactions:
DMARDs can interact with other medications. It’s important to inform healthcare providers of all medications, including over-the-counter drugs and supplements, to avoid potential interactions.
Severe Renal Impairment:
Some DMARDs, particularly certain conventional synthetic DMARDs, may have contraindications or require dose adjustments in individuals with severe renal impairment.
Neurological Disorders:
Certain DMARDs may be contraindicated in individuals with certain neurological disorders or conditions.
It’s important to emphasize that the decision to prescribe DMARDs is individualized and involves a careful assessment of the risks and benefits by a rheumatologist or healthcare provider. Before starting any DMARD, a thorough medical evaluation, including laboratory tests and imaging, is often conducted to identify potential contraindications and monitor for side effects. Patients are encouraged to communicate openly with their healthcare providers, providing information about their medical history and any concerns they may have to ensure safe and effective treatment.
Monitoring:
Patients must be tested for hepatitis B and C before beginning a DMARD. Furthermore, it is highly advised to get screened for tuberculosis before starting any biologic DMARD.
Some of these agents are teratogenic, whereas safety has not been proven in pregnancy for other agents. Pregnancy tests must be performed on women who are or may become pregnant before using these medications. Furthermore, any woman of childbearing age who uses these drugs, particularly leflunomide or methotrexate, needs to be using the recommended method of birth control.
While myelosuppression and hepatotoxicity can happen to a patient on a DMARD at any point, they are more common in the early stages of therapy. As a result, earlier in therapy, more frequent monitoring is advised; after early safety has been established, less frequent but regular monitoring must be maintained for as long as the patient is taking the DMARD.
Although hepatotoxicity and myelosuppression can occur at any time in DMARD therapy, they are more common in the initial phases of treatment. Therefore, it is recommended to monitor more frequently early in therapy; once early safety has been established, monitoring should be less frequent but still regular as long as the patient is taking the DMARD.
The lipid panel shall be monitored at baseline and then every 3 months for at least 6 months and then every 6 months thereafter in patients initiated on tocilizumab, tofacitinib, and sarilumab.
Patients receiving any of the biologic DMARDs must have their CBC checked every six months.
Close monitoring of renal function is also advised for patients on DMARDs every three to six months since many of these medications require dose adjustments in cases of renal insufficiency.
When patients are taking hydroxychloroquine, comprehensive ophthalmology exams are required at baseline, after five years, and then yearly. These exams should include visual field testing and ocular coherence tomography.
Toxicity:
High-dose methotrexate can result in transaminitis and severe pancytopenia in patients, particularly when given intravenously to treat certain cancers. Leucovorin rescue is utilized in these situations to stop methotrexate toxicity.
Classification of disease-modifying antirheumatic drugs:
Disease-modifying antirheumatic drugs (DMARDs) can be classified into different categories based on their mechanisms of action and chemical structures. Here is a broad classification of DMARDs:
Conventional Synthetic DMARDs (csDMARDs):
These are traditional DMARDs that have been in use for several decades. They are usually the first line of treatment for rheumatoid arthritis (RA) and other autoimmune rheumatic diseases.
- Methotrexate: Inhibits folate metabolism and has immunomodulatory effects.
- Sulfasalazine: Combines a sulfa drug with an anti-inflammatory agent.
- Hydroxychloroquine: Originally an antimalarial drug, it also has immunomodulatory effects.
- Leflunomide: Inhibits pyrimidine synthesis, affecting rapidly dividing cells, including immune cells.
Biologic DMARDs (bDMARDs):
These drugs are derived from living organisms or produced using biotechnology. They target specific components of the immune system involved in the inflammatory response.
Categories of biologic DMARDs include:
- Tumor Necrosis Factor (TNF) Inhibitors: Examples include adalimumab, etanercept, and infliximab.
- Interleukin Inhibitors: Examples include anakinra (IL-1 inhibitor), and tocilizumab (IL-6 inhibitor).
- B-cell Depleting Agents: Examples include rituximab.
- T-cell Co-Stimulation Inhibitors: Examples include abatacept.
Targeted Synthetic DMARDs (tsDMARDs):
These are synthetic drugs that target specific molecules involved in the inflammatory process. They offer an alternative to traditional csDMARDs and are used in conditions like RA.
- Janus Kinase (JAK) Inhibitors: Examples include tofacitinib, baricitinib, and upadacitinib.
Immunosuppressants:
Some medications with immunosuppressive properties are used as DMARDs, particularly in conditions like systemic lupus erythematosus (SLE).
- Cyclophosphamide: Alkylating agent affecting DNA synthesis.
- Azathioprine: Alters the proliferation of immune cells.
Glucocorticoids:
While primarily anti-inflammatory and used for symptomatic relief, glucocorticoids may be considered disease-modifying when used in low doses and short courses. Prolonged or high-dose use, however, is associated with significant side effects.
Examples include prednisone and prednisolone.
It’s important to note that the choice of DMARD depends on the specific autoimmune rheumatic disease, its severity, and individual patient factors. Additionally, combination therapies involving different classes of DMARDs or a combination of DMARDs and non-DMARDs may be used for optimal disease management. The selection of DMARDs is typically made by rheumatologists based on a comprehensive assessment of the patient’s condition. Regular monitoring is essential to assess treatment effectiveness and manage potential side effects.
Continuing education activities of DMARDs:
A class of medications known as disease-modifying antirheumatic drugs (DMARDs) is prescribed to treat a number of inflammatory arthritides, such as rheumatoid arthritis (RA), as well as other connective tissue disorders and certain cancers. The DMARDs’ mechanism of action, adverse event profile, pharmacology, monitoring, and pertinent interactions will be highlighted in this activity. This information is important for interprofessional team members treating patients with autoimmune disorders who will benefit from such therapy.
Objectives:
- Describe the distinctions between biological and conventional DMARDs.
- Determine the various DMARD agents’ indications.
- Describe the side effects and avoidance guidelines for biologic DMARD treatment.
- Explain the significance of enhancing interprofessional team care coordination to improve patient care when using biologic or traditional DMARDs.
Mechanism of action:
Every DMARD acts differently, eventually disrupting important pathways in the cascade of inflammation. For example, methotrexate suppresses cell-mediated immunity, inhibits synovial collagenase gene expression, decreases neutrophil adhesion, inhibits neutrophil leukotriene B4 synthesis, inhibits local IL-1 production, and lowers levels of IL-6 and IL-8. It also stimulates adenosine release from fibroblasts. Some drugs in this class work by preventing lymphocyte proliferation or by making them dysfunctional.
Leflunomide prevents the synthesis of pyrimidines by inhibiting dihydroorotate dehydrogenase, which in turn prevents the proliferation of lymphocytes. Sulfasalazine works against oxidative, nitrative, and nitrosative damage to mediate its anti-inflammatory effects. However, hydroxychloroquine, a very mild immunomodulatory drug, blocks the intracellular TLR9 toll-like receptor.
Conversely, biologics have an extremely selective mode of action. Biologics’ main actions consist of (1) interfering with the production or function of cytokines, (2) blocking the “second signal” necessary for T-cell activation, or (3) reducing the number of B-cells or preventing the factors that stimulate B-cells. The protein tyrosine kinase JAK, which is involved in mediating cytokine signaling, is inhibited by the small molecule tofacitinib.
Administration:
DMARDs (both biological and non-biologic) can be injected intravenously (IV), subcutaneously (SC), or orally.
How long does DMARDs take to work?
The time it takes for disease-modifying antirheumatic drugs (DMARDs) to show noticeable effects can vary widely among individuals and depends on several factors, including the specific DMARD prescribed.
Here are some general considerations:
Conventional Synthetic DMARDs (csDMARDs):
Drugs like methotrexate, a commonly prescribed csDMARD, may start to show some effect within a few weeks to a couple of months. However, their full disease-modifying potential may take several months to become apparent. It’s not uncommon for healthcare providers to monitor patients closely during the initial phase of treatment.
Biologic DMARDs (bDMARDs):
The onset of action for biologic DMARDs can vary. Some individuals may experience improvement relatively quickly, within a few weeks, while for others, it may take a few months. Response to biologics can be influenced by factors such as the specific drug used, the individual’s immune response, and the severity of the disease.
Targeted Synthetic DMARDs (tsDMARDs):
Drugs like Janus kinase (JAK) inhibitors, a type of targeted synthetic DMARD, may start to show effects within a few weeks. However, as with other DMARDs, the full benefit may take a few months.
Individual Response:
Response to DMARDs is highly individual. Some individuals may experience a rapid and robust response, while others may require more time. It’s important to be patient and communicate regularly with your healthcare provider about any changes in symptoms or potential side effects.
Combination Therapy:
In some cases, healthcare providers may prescribe a combination of DMARDs or a combination of DMARDs and other medications to enhance efficacy. This approach may expedite the onset of action and improve overall disease management.
Early Intervention:
Starting DMARD therapy early in the course of the disease is associated with better outcomes. Early intervention may help control inflammation, prevent joint damage, and improve long-term prognosis.
It’s essential to note that the goal of DMARD therapy is not just symptom relief but also the modification of the underlying autoimmune process. Therefore, even if symptoms improve, it’s important to continue DMARD treatment as prescribed by your healthcare provider.
Frequent monitoring and follow-up appointments are essential to evaluate the efficacy of the selected course of action and make any required modifications. If you have concerns about the timeline for improvement or experience side effects, it’s important to discuss these with your healthcare provider, who can provide guidance based on your specific situation.
How are disease-modifying antirheumatic drugs prescribed?
The prescription of disease-modifying antirheumatic drugs (DMARDs) is a complex process that involves careful consideration of the patient’s diagnosis, disease severity, medical history, and individual factors. Here is a general overview of how DMARDs are typically prescribed:
Diagnosis and Evaluation:
A diagnosis of an autoimmune rheumatic disease, such as rheumatoid arthritis (RA) or psoriatic arthritis, is typically made by a rheumatologist, or a specialized healthcare provider. The rheumatologist conducts a thorough evaluation, which may include a medical history, physical examination, blood tests, imaging studies, and assessment of symptoms.
Disease Severity and Progression:
The severity of the autoimmune disease and its potential for progression are crucial factors in determining the need for DMARD therapy. In some cases, the rheumatologist may assess the presence of joint damage or other systemic manifestations.
Selection of DMARDs:
Based on the diagnosis and disease characteristics, the rheumatologist selects the most appropriate type of DMARD. This decision may involve choosing between conventional synthetic DMARDs (csDMARDs), biologic DMARDs (bDMARDs), or targeted synthetic DMARDs (tsDMARDs). The choice may also depend on the patient’s preferences, lifestyle, and potential contraindications.
Combination Therapy:
In certain situations, especially when the disease is more severe, the rheumatologist may prescribe a combination of DMARDs or a combination of DMARDs and other medications (such as nonsteroidal anti-inflammatory drugs or corticosteroids) to achieve optimal disease control.
Individualized Treatment Plan:
The treatment plan is highly individualized, taking into account the patient’s unique characteristics, comorbidities, and potential risks and benefits associated with each medication. The rheumatologist discusses the proposed treatment plan with the patient, addressing any questions or concerns.
Monitoring and Adjustments:
Once treatment begins, regular monitoring is essential. This may involve follow-up appointments, blood tests, and imaging studies to assess the effectiveness of the chosen DMARD and to monitor for potential side effects. Adjustments to the treatment plan may be made based on the patient’s response and any changes in disease activity.
Patient Education:
Patient education is a critical component of DMARD therapy. Patients are often provided with information about the importance of medication adherence, potential side effects, and the need for ongoing monitoring. This empowers patients to actively participate in their treatment and communicate effectively with their healthcare team.
What are the common side effects of DMARDs?
Disease-modifying antirheumatic drugs (DMARDs) can have various side effects, and the specific side effects may vary depending on the type of DMARD prescribed. It’s important to note that the benefits of DMARD therapy often outweigh the potential risks, and healthcare providers carefully consider the risk-benefit profile when prescribing these medications. Here are some common side effects associated with different types of DMARDs:
Conventional Synthetic DMARDs (csDMARDs):
Methotrexate:
- Common side effects include nausea, fatigue, mouth sores, and gastrointestinal upset.
- Long-term use may be associated with liver function abnormalities.
- Regular monitoring of blood counts, liver function, and kidney function is usually recommended.
Sulfasalazine:
- Nausea, vomiting, and diarrhea are typical gastrointestinal side effects.
- Skin rash and headaches can occur.
- It is frequently advised to regularly monitor liver function and blood counts.
Hydroxychloroquine:
- Gastrointestinal upset, including nausea and diarrhea, may occur.
- Eye problems, including retinopathy, are potential but rare side effects.
- It is advised that patients receiving long-term therapy have regular eye exams.
Leflunomide:
- Diarrhea, nausea, and liver enzyme elevation are common side effects.
- Leflunomide has a long half-life, and a washout procedure may be necessary if side effects occur.
- Blood pressure should be monitored regularly.
Biologic DMARDs (bDMARDs):
TNF Inhibitors (e.g., infliximab, etanercept, and adalimumab):
- Injection site reactions are common.
- Increased risk of infections, particularly respiratory and skin infections.
- Rare but serious side effects include demyelinating disorders and an increased risk of certain malignancies.
- Monitoring for signs of infection and regular screening for tuberculosis is essential.
Interleukin Inhibitors (e.g., tocilizumab, anakinra):
- Increased risk of infections, including upper respiratory infections.
- Changes in liver enzymes may occur.
- Risk of gastrointestinal perforations with certain medications.
- Routine monitoring of blood counts, liver function, and cholesterol levels may be recommended.
B-cell Depleting Agents (e.g., rituximab):
- Increased risk of infections, including reactivation of viral infections.
- Infusion reactions, including rash and respiratory symptoms.
- Long-term effects on immunoglobulin levels.
- Monitoring for infections and routine immunoglobulin level assessments may be necessary.
Targeted Synthetic DMARDs (tsDMARDs):
Janus Kinase (JAK) Inhibitors (e.g., tofacitinib, baricitinib):
- heightened susceptibility to infections, particularly upper respiratory infections
- Changes in liver function.
- Potential for increased lipid levels.
- Routine monitoring of blood counts, liver function, and lipid levels may be advised.
It’s crucial for individuals taking DMARDs to communicate openly with their healthcare providers about any side effects or concerns. Regular monitoring, as recommended by the healthcare team, helps manage and mitigate potential risks associated with these medications. Always follow the prescribed treatment plan and attend scheduled follow-up appointments to ensure the safe and effective use of DMARD therapy.
Can DMARDs cause allergic reactions?
Yes, disease-modifying antirheumatic drugs (DMARDs) can potentially cause allergic reactions in some individuals. Allergic reactions can range from mild to severe and may manifest as skin rashes, itching, swelling, difficulty breathing, or anaphylaxis, which is a severe and potentially life-threatening reaction.
The likelihood of an allergic reaction to a DMARD varies among individuals and is influenced by factors such as:
Individual Sensitivity:
Each person’s immune system reacts differently to medications. Allergy reactions might be more common in certain people.
Specific DMARD:
Different DMARDs have distinct chemical structures and mechanisms of action. Allergic reactions are more common with certain drugs.
Drug Class:
Biologic DMARDs, which are derived from living organisms, may pose a higher risk of allergic reactions compared to conventional synthetic DMARDs. However, allergic reactions can still occur with any type of DMARD.
Previous Allergic History:
Individuals with a history of allergies, especially to medications, may be at a higher risk of developing allergic reactions to DMARDs.
Administration Route:
Allergic reactions can occur with both oral and injectable forms of DMARDs. Injectable forms, particularly biologics administered by subcutaneous or intravenous injection, may have a higher risk.
It’s important for individuals taking DMARDs to be vigilant for signs of an allergic reaction and to seek immediate medical attention if any symptoms occur.
Common signs of an allergic reaction include:
- Skin rash or hives
- Itching
- Swelling of the face, lips, or tongue
- Difficulty breathing or shortness of breath
- Chest pain or tightness
- Severe dizziness or fainting
If an allergic reaction is suspected, it’s crucial to stop taking the medication and seek emergency medical attention promptly. Healthcare providers should be informed about any known allergies or previous adverse reactions to medications during the prescribing process.
Enhancing healthcare team outcomes:
Rheumatoid arthritis (RA) and numerous other autoimmune disorders, such as systemic sclerosis, vasculitis, spondylarthritis, inflammatory myositis, inflammatory bowel disease, systemic lupus erythematosus, and certain cancers, can be treated with disease-modifying antirheumatic drugs (DMARDs).
Owing to the considerable intricacy associated with using these agents appropriately and keeping an eye out for side effects, DMARDs should ideally be prescribed by a qualified professional, such as a dermatologist, gastroenterologist, or rheumatologist. In addition to coordinating with the pharmacist regarding dosing, possible interactions, and adverse events, the prescribing provider must be knowledgeable about these agents, their indications, and their side effects.
Since all patients prescribed DMARDs need to have their side effects and effectiveness closely monitored, nurses can also counsel patients and work in tandem with the pharmacist and clinician to monitor the patient. With the fewest adverse events, the interprofessional approach will offer the best opportunities for favorable patient outcomes.
Precautions to take when using DMARDs:
Using disease-modifying antirheumatic drugs (DMARDs) requires careful management and monitoring to ensure both effectiveness and safety. Here are some precautions to consider when using DMARDs:
Regular Monitoring:
Regular monitoring is essential to assess the effectiveness of the DMARD and to detect and manage potential side effects. This may include blood tests to check liver function, kidney function, blood cell counts, and other relevant markers.
Communication with Healthcare Providers:
Maintain open and regular communication with your rheumatologist or healthcare provider. Notify someone right away if your symptoms, side effects, or concerns change.
Compliance with Prescribed Treatment:
Adhere to the prescribed treatment plan and take the medication exactly as directed by your healthcare provider. Missing doses or stopping treatment without guidance can affect the effectiveness of the DMARD.
Patient Education:
Educate yourself about the medication you are taking, including potential side effects, drug interactions, and the importance of regular monitoring. Understanding your treatment plan will empower you to actively participate in your care.
Infection Prevention:
DMARDs, especially biologics and other immunosuppressive medications, can increase the risk of infections. Practice good hygiene, and promptly report any signs of infection to your healthcare provider. Stay up-to-date on vaccinations, but avoid live vaccines while on immunosuppressive therapy.
Dental Health:
Some DMARDs, particularly those that affect the immune system, may increase the risk of dental infections. Maintain good oral hygiene, and inform your dentist about your medication before dental procedures.
Liver Health:
Certain DMARDs, such as methotrexate, can affect the liver. Avoid excessive alcohol consumption and inform your healthcare provider of any history of liver disease.
Pregnancy and Contraception:
Discuss family planning with your healthcare provider, as some DMARDs may have implications for pregnancy. Some medications may need to be adjusted or stopped before conception. Effective contraception may be necessary for individuals of childbearing age.
Eye Health:
For individuals taking hydroxychloroquine, regular eye examinations are important to monitor for potential retinal toxicity.
Bone Health:
Long-term use of corticosteroids, which are sometimes prescribed in conjunction with DMARDs, can affect bone health. Adequate calcium and vitamin D intake and weight-bearing exercise are important considerations.
Medication Adjustments:
If you experience side effects or your disease activity changes, consult your healthcare provider before making any adjustments to your medication.
Always consult your healthcare provider for personalized advice and guidance based on your specific medical history and the type of DMARD prescribed. The precautions may vary depending on the specific DMARD and individual health factors. Regular follow-up appointments with your rheumatologist are crucial for ongoing assessment and adjustments to your treatment plan.
FAQs:
Methotrexate is now considered the first-line DMARD agent for most patients with RA.
No, DMARDs aren’t steroids. DMARDs are medications that preserve the immunomodulatory effects of steroids without having the systemic side effects associated with corticosteroids. Weight gain, bruising easily, elevated blood pressure, and blood sugar are some of these adverse effects.
Osteoarthritis (OA) is not treated with conventional DMARDs; they are only used to treat inflammatory arthritis.
People who are suffering from active infection, leukopenia, blood cancer, and immunodeficiency disorders are not allowed to take DMARDs.


2 Comments