Epidural Fibrosis
Table of Contents
What is Epidural Fibrosis?
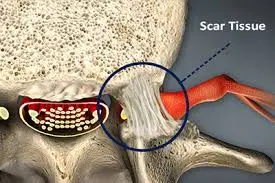
Epidural fibrosis, also known as peridural fibrosis or epidural scarring, is a condition that occurs as a result of the healing process after spinal surgery or spinal trauma. It refers to the formation of excessive scar tissue in the epidural space, which is the area surrounding the spinal cord and the spinal nerves.
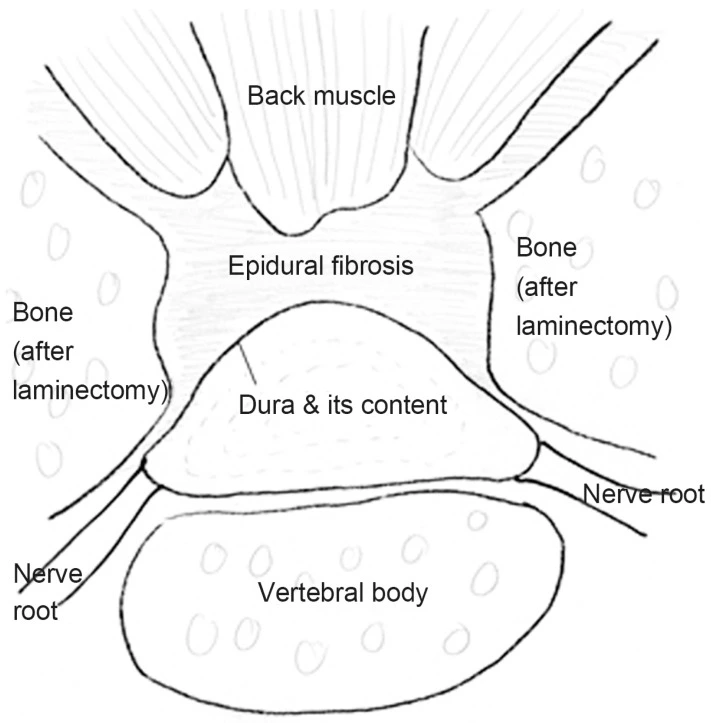
In epidural fibrosis, fibrous scar tissue restores the epidural fat and can cause contraction of the dura mater and nerve roots, leading to their adherence and tethering to the adjacent structures
Scar tissue that develops following back surgery is known as epidural fibrosis. Failed back surgery syndrome (FBSS) is a disorder with a number of potential causes. Perhaps the most typical cause of FBSS is epidural fibrosis.
However, there is good news: Pain or other symptoms are not a constant sign of epidural fibrosis. In fact, for some people, it has no impact at all on their regular activities or pain levels. The extent of the scarring may have an impact on whether or not symptoms manifest, according to a 2015 study that was reported in the journal Insights Imaging.
Another study from 2015 indicated that up to 36% of persons with failed back surgery syndrome may have discomfort due to epidural fibrosis. This study was published in the Asian Spine Journal.
Furthermore, while 36 percent of patients is a significant chunk, 91 percent is still a long way off.
Epidural fibrosis can develop after back surgery and is comparable to but distinct from arachnoiditis, a rare persistent pain syndrome. First, whereas arachnoiditis penetrates deeper into the arachnoid membrane, epidural fibrosis affects the dura mater, the outermost covering of the spinal cord. The arachnoid envelops and guards the delicate nerves that make up the spinal cord, just like the dura mater above it and the pia mater below it.
Another distinction is that although back surgery is one of several potential causes of arachnoiditis, epidural fibrosis is brought on by back surgery. Finally, inflammation may result in the formation of scar tissue, which may ultimately result in the clumping of the spinal nerves—a painful disease that is challenging to cure.
Epidemiology and Etiology
Approximately 40% of lumbar laminectomy patients experienced FBSS, and 4% to 9% of patients required a second operation.
Although the exact cause of FBSS is unknown, numerous investigations concur that it has a complex origin and that its causes may be divided into preoperative, operative, and postoperative components. Preoperative variables may also be further subdivided into patient-related variables such as psychiatric and social issues and surgery-related variables such as bad candidate selection, revision surgery, and faulty planning. Operative variables include inappropriate level surgery (2.1%–2.7%), excessive decompression with foramina instability, and insufficient decompression of lateral recesses. Recurrent disc herniation (6%–23%), neighboring segment illness, issues with sagittal balance, pelvic incidence, lumbar lordosis mismatch, battered root syndrome (20%–36%), and nerve root entrapment syndrome are postoperative risk factors. Leg and back pain are mostly caused by the extension of scar tissue into the neural canal and adherence to the dura mater.
Formation
When you get epidural fibrosis, what exactly occurs to your spine? This response often has to do with the spinal nerve root, a region of your spine.
The two procedures most frequently performed to treat back and leg pain are laminectomy (also known as decompression surgery) and discectomy. The goal of these treatments is to release the pressure that is placed on the spinal nerve root as it leaves the spinal cord. (Different structures, such as disc fragments or bone spurs pushing on and irritating the nerve root, can be caused by injuries such as herniated discs as well as degenerative changes in the spine itself.)
This implies that a spine surgeon would often operate close to where your nerve root is located. They may need to excise them with a sharp tool since their attention will be on getting rid of items (such as disc pieces that don’t belong there or bone spurs that are too close to the nerve). Because of this, your procedure will result in the formation of a wound.
Any trauma that alters a physical structure naturally results in scarring, and the region around your spinal nerve root following surgery is no exception. In other words, the growth of epidural fibrosis is identical to the scab that grows on your knee after the original damage. The process is similar to what occurs when you scratch your knee. Both epidural fibrosis and the scab are organic healing processes.
Epidural scarring often appears six to twelve weeks following surgery.
Pathophysiology
Let’s explore this healing process in more detail as it relates to your discectomy or laminectomy. Several things can occur “under the hood,” so to speak, after back surgery.
First, the dura mater, the outermost of your spinal cord’s three protective coverings, may get crushed. Second, a nerve root or root may end up being “tethered” or knotted. Thirdly, the blood supply to the nerve root and/or cerebral spinal fluid is blocked as a result of one, the other, or both of these factors.
Between the arachnoid mater and the pia mater, the cerebrospinal fluid (CSF), a transparent, watery fluid, passes between the brain and spinal cord. Its function is to absorb shock and shield the brain and spinal cord, the only components of the central nervous system, from damage.
In 2016, experts were still arguing whether or how scarring on or close to the spinal nerve root correlates with the pain and other issues you might mention to your healthcare practitioner after having back surgery. According to the aforementioned Asian Spine Journal article, other study writers disagree, claiming that there is no connection between the two. Others, however, according to the Asian Spine Journal, have come to the conclusion that there is a connection between symptoms and discomfort and broad scarring in and around the nerve root (as opposed to fibers that are identified in just one region).
In any case, there is no really efficient therapy once the scars have formed. Your surgeon might want to return and use an endoscope to break up the scars, but doing so could cause additional scarring and epidural fibrosis.
For this reason, preventing or at the very least reducing the development of the scar is the best strategy to treat epidural fibrosis.
Currently, research investigations are being conducted to determine how to accomplish it, primarily on animals rather than people. These studies mostly use rats to test medications or materials before comparing the tissues to a control group (rats who don’t get the drugs or materials).
Three stages can be distinguished in the development of scar tissue:
- The first stage occurs between the third and fifth day following surgery and is characterized by a local inflammatory reaction, primarily involving the hemostasis and coagulation processes as well as the release of chemokines like phospholipase A2, which causes the aggregation of macro phagocytes, fibroblasts, mastocytes, and endotheliocytes. A hematoma totally fills the laminectomy’s bone breach and is in contact with the muscle’s deep surface. A variable distance below the undamaged neural arcs above and below the defect surgery, the hematoma borders the posterior and lateral dura surfaces. After a week, we see significant fibroblastic activity on the deep surface of the muscle, and these cells appear to track the hematoma’s growth.
- The second phase, which lasts for two to three weeks, is characterized by the proliferation and differentiation of fibroblasts into fibrocytes, the gradual formation of granulation tissues, and the secretion of collagenous fibers into the defect lesion. These processes are controlled by cytokines like transforming growth factor (TGF)-b1, interleukin-6 (IL-6), and fibroblast growth factor (FGF). Additionally, TGF-b1, IL-6, and FGF-2 may be secreted by fibroblasts to promote fibroblast proliferation and extracellular matrix formation. Inflammatory mediators including interleukin 6, interleukin 8, and TNF that were present in the disk were in fact released in animal tests. They have a thick, fibrous scar from the erector muscle that reaches the spinal roots along the dura’s lateral wall around the age of 15 days. The scar continues into the conjugation foramen if the laminectomy is extended up to it. The bone defect is filled in this fashion by a thick, resilient membrane that clings tenaciously to the dura and root beneath.
- The third phase, tissue rebuilding, lasts from months to years and involves the deposition of fibrillary connective tissues surrounding the defect lesion and their subsequent conversion to scar tissues.
Four explanations have been put out in the literature to account for the origin of fibrosis:
- The earliest researchers on this issue, Key and Ford, came to the conclusion that the major cause of fibrosis is damage to the disc’s fibrous ring during the surgical process to remove the ruptured disc. Instead, they asserted that the anterior area was the primary cause of the scar: curettage of the annulus would cause fibroblasts to become activated and spread along the hematoma that inevitably occurs in the anterior section of the canal as a result of discectomy (anterior-lateral fibrosis). This anterior hemorrhage normally heals more rapidly than the posterior one; as a result, the scarring in the anterior area, which causes roots and dura to adhere to the surface of the annulus, is often less widespread.
- The inferior interested surface of the erector muscle of the spine, which comes into touch with the dura through a surgical bone defect, is thought by LaRocca and Macnab to be the main cause of fibrosis. A thick and long-lasting membrane called the “laminectomy membrane” covers the surgical opening from the sacrospinalis to the side of the dura mater (posterior-lateral fibrosis) by means of the proliferation of fibroblasts in the deep layer of the sacrospinalis.
- Hoyland believed that the formation of fibrosis was caused by the retention of cotton particles that broke off from the surgical swabs employed. When epidural scar samples from individuals who had recent surgery were examined under a polarized microscope, bi-reflective crystalline deposits that are occasionally phagocytized by macrophages were found; these deposits were surrounded by a small number of persistent infiltrates of inflammatory cells. It was proven using histochemical and enzyme histochemical techniques that this substance is derived from the surgical swabs since it is birefringent and correlates to both cotton fibers and pure cellulose.
- The scar tissue that surrounds the dura arises not only from the front of the sacrospinalis muscle but also from the back of the annulus fiber and posterior longitudinal ligament, according to a three-dimensional theory put out by Songer and Ghosh Spencer. Epidural adhesion is brought on by the hyperplasia of fibrous tissue around the ventrolateral nerve root. Songer, in particular, supports the idea that the irritating substance from the nucleus pulposus leftovers drives the development of fibrosis.
The majority of authors concur that the area of the paravertebral muscles that come into contact with the dura after laminectomy is the primary site of fibrosis; the size of the surgical bone defect and the presence of blood clots in the epidural space are factors that contribute to the scar’s extension. The formation of scar tissue in the anterior or anterolateral epidural space and the role that irritating material from the nucleus pulposus entering the annulus fibrous through surgical defects in the annulus and the Longitudinal Posterior Ligament (LLP) can play in its development has recently received more attention. A2 phospholipases are abundant in the nucleus pulposus, where they activate the cascade of arachidonic acid to create the prostaglandins E1, E2, and leukotrienes B. These compounds significantly reduce the stimulation threshold of C fibers, which are prevalent in nerve roots and cause inflammation when stimulated.
After the discectomy, the residues of the nucleus pulposus containing high levels of phospholipase A2 may come into contact with the dura and root through the defect in the annulus and the LLP, resulting in a chronic inflammation at the level of the discectomy, producing PGE with the subsequent release of the cytokines from leukocytes. At the level of the anterior lateral epidural space, the cytokines stimulate the growth of fibroblasts, the creation of collagen, and subsequently the development of scar tissue. PGE2 may also work directly to cause the development of scar tissue. The creation of epidural scars is posterior and anterior for each interlaminate method (laminectomy and discectomy), according to the information above: The extent of surgical dissection and the width of the surgical bone defect both directly affect how far it extends. Large hematoma and imperfect hemostasis encourage tissue fibrotic epidural expansion over the boundaries of the bone defect.
Degree of Fibrosis
The degree of fibrosis is one factor that research has connected to symptoms and pain. There are many grades of epidural fibrosis, ranging from Grade 0 (which reflects normal tissue with no scarring at all) to Grade 3. When a laminectomy was performed, grade 3 fibrosis is characterized by scar tissue that occupies more than two-thirds of the surgical site. While Grades 1 and 2 do not, a Grade 3 scar may potentially reach the nerve root. Scars of Grade 3 more closely resemble symptoms and discomfort than those of Grades 1 and 2.
The dura mater, the outermost spinal cord covering mentioned above, is where scars of grade 1 are often put down. These scars are typically minor and composed of thin fibrous bands. Less than two-thirds of the laminectomy region had moderate, continuous Grade 2 scars. When a scar reaches Grade 2, it is continuous; therefore, it is difficult to see separate strands.
Neuro-mechanic hypothesis
In healthy individuals, the spinal cord may move upward or downward in conjunction with the spinal flexion, extension, and abduction of the lower limbs as well as the flexion, extension, and lateral flexion of the trunk. The dura and the roots are tractioned by these motions, which do not hurt. When epidural fibrosis is present, the roots and dura are tenaciously attached to the scar tissue; as a result, movements of the limbs and trunk cause deformation on the roots and dura and the emergence of discomfort.
Additionally, even if the scar was not the cause of the pain directly, its presence in the epidural space would result in a state of relative constriction of the canal or foramen, for which tiny protrusions or osteophytes would also become symptomatic. The epidural scar is thus more extensive, which is why the patient is in agony. Instead, gadolinium-enhanced MRI investigations revealed no discernible differences between symptomatic and asymptomatic individuals in the volume and extent of scar tissue. Additionally, this mechanical hypothesis is unable to explain why a second operation for the neuro-lysis of scars only temporarily reduces pain.
The hypothesis of intra-neural fibrosis and demyelination
A rupture of the blood-brain barrier in the endoneurial space, followed by root edema and increased endoneurial liquor pressure, is brought on by the chronic root compression brought on first by disc herniation and then by fibrosis. This leads to the demyelination of large-diameter fibers and the development of dura fibrosis and arachnoid thickening.
The nucleus pulpous’s release of chemical mediators is hypothesized to occur
After discectomy, there is a hypothesis that the nucleus pulpous continued to emit chemical mediators. The nucleus pulpous remnants are situated adjacent to the dural sac and the roots following the discectomy through the annulus fenestration. These residues have high A2-phospholipase concentrations, which cause the arachidonic acid cascade to be activated and produce leukotrienes B, El, and E2 in the epidural space. These chemicals lower the threshold for stimulation or directly excite the type C small-diameter fibers, which are located inside the roots and are responsible for transmitting pain.
Diagnosis
A magnetic resonance imaging (MRI) scan may be prescribed by your doctor to identify any epidural fibrosis you may have. The issue is that with this kind of diagnostic imaging examination, scarring is frequently invisible. Therefore, you might also need to have an epiduroscopy if you experience symptoms yet the results of your MRI are negative.
An epiduroscopy is a test that lets your surgeon view what’s happening at your nerve root by inserting a probe, or scope, into the problematic location. It’s critical to complete the diagnostic procedure since your discomfort may not truly be caused by epidural fibrosis but rather by another disc herniation. If this were the case, you would probably require additional surgery; but, if the epiduroscopy results revealed scarring and the scarring was the root of your issues, you probably wouldn’t.
Prevention of Epidural Fibrosis
The fibroblasts surrounding the surgical environment are carried into the operating region by blood, which is one potential mechanism for the creation of the epidural scar. Therefore, limiting fibroblast migration, ensuring complete hemostasis in the operative region, and isolating the interaction between the spinal dura mater and fibrous tissue are the key prevention strategies for epidural fibrosis. The use of interposition materials and proper surgical technique are the two key components that prevent the development of epidural scars.
Correct surgical technique, which includes retaining periradicular fat, meticulous curettage of the inter somatic space, proper hemostasis of the space, and anatomical rebuilding of the periradicular space, is a precondition for preventing the scar from forming. Bipolar coagulation, little nerve root retraction, and effective hemostatic control are essential. the use of drugs like Mitomycin C, dexamethasone, hydroxy camptothecin, rosuvastatin, nonsteroidal anti-inflammatory drugs, low dose radiation, conventional Chinese medicines, and biomaterials like autologous tissue and biodegradable polymeric materials are some of the current clinical techniques currently being used.
The development of fibrotic tissue in the epidural area is prevented or minimized by sandwiching an inert material between the dura, muscles, and annulus. Different interposition materials have been investigated; some have only been utilized in experiments, while others have been applied in clinical settings. These materials are separated into biological and non-biological (porous or not porous) categories. Examples of biological materials are free or pedunculated fat grafts, flavum ligament, and dura mater. The non-porous materials may be separated into solids like Dacron, Poly-methyl-methacrylate, expanded polytetrafluoroethylene membranes, and viscous materials like hyaluronic acid, carbohydrate polymer gel, or carboxy-methyl-cellulose gel. The porous materials include Gelfoam, Avitene, and bone wax.
- Biological materials: In surgical practice, free or pedunculated fat grafts are frequently utilized to stop or slow the development of epidural scars. However, there are certain issues associated with their use: because the fat does not completely cover the laminectomy incision, fibrotic tissue enters the gap through the borders. His saponification may squeeze the cauda if you utilize too much fat. At the conclusion of the procedure, it was suggested to form the peduncle of the flavum ligament and move it between the two hemilaminas. Laminectomy procedures have included dural patches, however, they must be secured with sutures at the borders of the bone else dislocation is a typical occurrence.
- Non-biological materials: Because it appears that the porous materials (Gelfoan, Avitene) operate as a framework for the formation of fibrotic tissue or even seem to promote it, they are not an effective method of interposition. On the other hand, non-porous materials serve as a strong anti-fibrous barrier. The majority of non-porous solid materials take the form of membranes and must be fixed at the laminectomy margins. As a result, there is a risk that scar tissue will enter the epidural space through the gap between the membrane and the edges of the operated bone. Viscous materials are arguably the best type of substance. Due to their viscosity, they can be injected into both the anterior and posterior epidural space at the level of the bone defect anterior to the dura and the roots, laterally in the lateral recess, and posteriorly in the bone defect between the dura and the posterior spinal muscles: In doing so, they avoid the development of fibrosis posteriorly and posterior-laterally to the roots and dura. Additionally, they are biocompatible due to their total absorption, even if it happens gradually (4 weeks on average).
- They also affect the extracellular control of inflammatory cell migration: in vitro, they block lymphocyte, macrophage, and granulocyte migration as well as fibroblast mobility and multiplication and cytokine generation. Polymeric materials might be categorized as either natural or manmade polymeric materials. Chitosan, fibrin gel, hyaluronate, and amniotic membrane are examples of natural polymeric materials that have been proven to work, although their effectiveness has been restricted. For instance, gelatin sponges can produce hematomas after expanding due to blood absorption, and they eventually change into scar tissue with epidural adhesion.
In order to suit the anomalous half elliptical dura mater correctly, a cross-linked hyaluronate must be semifluid. This reduces the survivability of NIH/3T3 fibroblasts, downregulates the expression of S100a and P4hb in NIH/3T3 fibroblasts, and inhibits the development of scar tissue in vivo.
- A chitosan-silane membrane: A chitosan-silane membrane has increased mechanical strength, which enables it to keep a certain form and fend off adhesion.
- The amniotic membrane: The inner layer of the fetal membrane known as the amniotic membrane serves as a barrier to lessen swelling, prevent vascularization, and restrict postoperative adhesion. Comparing it to a fat graft in a rat model, it can be beneficial to lessen adhesion since it has greater biocompatibility and the capacity to last for a specific amount of time in the body.
- Polylactic-co-glycolic acid membrane (PLGA): On the rabbit, it was shown that PLGA is a membrane with excellent blood compatibility, low organization response, precise barrier function, and a moderate absorption cycle. However, because PLGA is an absorbable material, there will be a cavity after PLGA absorption. There is a thermos-gel composed of PLGA and PEG that works as a support and an efficient, well-modulated barrier device to avoid postoperative adhesion. PEG is biodegradable, and when combined with PLGA, a material with good results but no cytotoxicity is created.
- ADCON-L: ADCON-L is a bioabsorbable carbohydrate polymer gel that is composed of a poliglycan ester and porcine-derived gelatin in phosphate-buffered saline and decreases postoperative fibrosis as seen in MRI studies. After its use, it has been strongly criticized in systematic reviews for its negative influence on wound healing and bone fusion, increased rate of CSF leakage, and collateral effects such as pain, non-CSF fluid collections, tachycardia, and erythematous skin reactions.
- e-PTFE: The expanded polytetrafluoroethylene membrane (e-PTFE) is highly biocompatible and structurally stable, preventing fibroblast penetration. It is made of porous materials that are superior at maintaining biocompatibility and a stable posture.
- MAACP/nHa multi-amino acid copolymer/ nanohydroxyapatite: Because HA is a component of osseous tissue, MAACP-nHA has excellent biocompatibility. Because the degradative product is neutral, it does not induce aseptic inflammation or displacement of the artificial lamina.
There are several ways to combine the benefits of medications like dexamethasone, ibuprofen, mitomycin C, and hydroxy camptothecin to create a physical barrier.
- Gelatin sponge+dexamethasone: Gelatin sponge performs an interval barrier effect, separating the nervous tissue from the surrounding tissue. Dexamethasone, an anti-inflammatory, stops the first and second stages of hematoma formation, delays granulation formation to prevent adhesion, reduces scarring, and prevents adipocyte necrosis.
- Fibrin glue+methylprednisolone acetate: It is a macromolecule that can create a network structure to inhibit the multi-bioactivity protein from bleeding and has a higher rate of in vivo disintegration without bacterial infection. It is a substance that is physiologically compatible, nontoxic, and non-reactive.
- Mitomycin C+ PEG film/PLGA film: PEG or PLGA were linked to mitomycin; in the former, PEG lowers the content of hydroxyproline. In the early wound of a post-laminectomy rat model, it has been demonstrated that mitomycin C-PEG controlled-release film inhibits collagen production and promotes the death of fibroblasts; only high concentrations of mitomycin C are cytotoxic.
- Ibuprofen-conjugated hyaluronate (HA)/polygalacturonic acid (PGA) hydrogel: Ibuprofen reduces prostaglandin E2 and the lipopolysaccharide synthesis that is produced in RAW264.7 cells, and thereby postpones the condensing of scar tissue.
- Carboxy-methyl-cellulose oly-ethylene oxide: Compost made of sodium carboxymethylcellulose and polyethylene oxide, CMC/PEC (Oxyplex) prevents fibrosis by preventing the growth of fibrin tissue on the area it covers. The first component possesses qualities that allow it to adhere to tissues. In 30 days, the gel is removed from the epidural space. This appears to be quite secure.
- PG (Duraseal exact): PG is a synthetic hydrogel that works as a barrier between tissues, is absorbable in two months, and is mostly made of polyethylene glycol.
A kind of autologous stem cell is an ADSC. They exhibit simple accessibility, relative abundance with the capacity for proliferation, and simple differentiation to fat tissue as compared to bone marrow stem cells. The scaffold should, in the meanwhile, meet the requirements outlined above, and the rate of degradation should be consistent with the rate at which ADSCs proliferate and differentiate. Soft scaffolds may be preferable to hard scaffolds since the material should be able to adhere everywhere it may occur in order to restrict the invasive fibroblast. In the early stage, soft scaffolds serve as a physical barrier by filling every available space in the fault region. The modified adipose tissue will subsequently operate as a bio-barrier once the stem cells develop into adipose tissue. Further research should identify better-suited and efficient materials and methodologies for the created adipose tissue.
Treatment of Epidural Fibrosis
You might be wondering what to do if a follow-up operation does not seem to be able to ease your epidural fibrosis discomfort.
This specific cause of failed back surgery condition still lacks a viable therapy, according to researchers and medical professionals. But often, medicine is initially administered, frequently in combination with physical therapy. Most likely, the drug will lessen the discomfort and make the activity bearable. A number of medications are administered, including gabapentinoids, non-steroidal anti-inflammatories (NSAIDs), and Tylenol (acetaminophen).
Physical therapy might include strengthening, stretching, and core exercises and is intended to keep you mobile. Maintaining joint mobility may prevent the production of scar tissue.
According to one study, the success rate of surgery is often only between 30% and 35%. Furthermore, according to the same study, up to 20% of patients’ symptoms actually get worse. Percutaneous adhesiolysis and spinal endoscopy are the two major surgical procedures used to treat epidural fibrosis.
Percutaneous adhesiolysis currently provides the strongest supporting data. The drug, which is frequently steroidal medication, is administered into the region using a catheter that has been put in during this treatment, which is, by the way, utilized for other reasons of failed back surgery syndrome as well. Additionally, with this surgery, symptom reduction does not need mechanical scar break-up.
Level I evidence (the highest quality) supports the use of percutaneous adhesiolysis for the symptoms of failed back surgery syndrome in general, including epidural fibrosis.
Your healthcare practitioner could also recommend spinal endoscopy as a therapy. A scope that enables your healthcare practitioner to see the region is placed during this operation. Spinal endoscopy is classified as having Level II and III evidence, and according to one research, there is “fair” evidence that it can relieve symptoms.
Medical and surgical interventions are two viable methods for treating pain brought on by the onset of epidural fibrosis. Muscle relaxants, opioids, and their derivatives, antidepressants, anti-epileptic medications, and spinal infiltrations are among the medical therapies available.
Surgery options include spinal cord stimulation (SCS), spine fusion and grafting to restore sagittal and coronal spinal balance, and reoperation to remove epidural scar tissue.
According to the gate control theory of pain, spinal cord stimulation (SCS) is thought to relieve pain by activating dorsal column A fibers, as this breaks up pain impulses that are sent from the periphery and are carried by C fibers and A-delta fibers. The common nerve synapse’s position in the substantia gelatinosa of the dorsal horn facilitates this disruption. Or, to put it another way, activation of the touch and vibration nerves “closes the gate” on ascending pain impulses that transmit noxious pain sensations cephalad, having varying impacts on sensory and pain thresholds as well as measurably changing higher-order cortical processing.
The adoption of completely implanted devices and long-term usage will maximize this impact. The early gate control theory-based mechanism of action is still not fully understood. SCS most likely functions by inhibiting nociceptive message transmission and nociceptive neuron excitation in the dorsal horn of the spinal cord as well as by activating inhibitory descending pathways and other supra-spinal processes. Percutaneously or surgically, the stimulation electrode is inserted into the posterior epidural space and in close proximity to the spinal cord. There are two steps to the implant of the stimulation system: trial stimulation and definitive implant and chronic stimulation.
Conclusion
Before attaching the electrode to a subcutaneously implanted stimulator, the trial stimulation phase—using an external battery for a few days (about 7 or 15 days)—must be completed in order to evaluate the effectiveness of the therapy (pain intensity drop >40%–50%). Only tonic (50-90 Hertz) continuous stimulation that causes observable paresthesia has been employed for more than 40 years. In order to prevent the sensation of paresthesia or improve pain relief, novel stimulation methods, such as burst stimulation, high-frequency (>1000 Hertz) stimulation, or high-density stimulation, have recently been proposed. The indications for SCS have been outlined in a number of consensus guidelines, and they mostly deal with neuropathic pain following the failure of traditional pharmacological therapies. The most prevalent symptoms are pain from peripheral neuropathy, complicated regional pain syndromes, and post-operative persistent low back and radicular pain (FBSS).
FAQs
Epidural fibrosis, or scarring, is the body’s normal reaction to this incision. When this scarring manifests as anticipated, the healing process proceeds normally. The nerve can start to send pain signals with even the smallest motions, though, if this scar tissue encloses or compresses the surrounding nerve root.
Epidural fibrosis treatment focuses on reducing discomfort and reducing inflammation. Depending on the degree of discomfort, doctors frequently suggest over-the-counter or prescription drugs. Vitamins and antioxidants are helpful in lowering inflammation close to the nerve root that causes pain.
Laser therapy: Laser radiation may also disintegrate fibrous tissue and support the formation of collagen, which helps smooth the skin’s surface. Surgery: To remove the fibrous tissue and restore a smooth skin surface in extreme instances, surgery may be required.
If scar tissue is the source of the discomfort, the pain and inflammation are reduced when a catheter is implanted and medicine is given to eliminate the scar tissue. If additional space is required around the compressed nerve, a balloon can be introduced.
Fibrosis is one of the most often occurring postoperative side effects. It is frequently seen in the days or weeks following surgery or in the recovery period. You may first feel small lumps on the scar tissue; but, as time passes, you’ll notice that they thicken and become painful.
Following back surgery, the development of scar tissue close to the nerve root, also known as epidural fibrosis, is a frequent occurrence. This is true both for patients with favorable surgical results as well as for those who experience ongoing or recurring leg discomfort and back pain.
There is a 1 in 1,000 to 1 in 100,000 chance of nerve injury. after many of these situations, the symptoms become better or go away after a couple of weeks or months. An uncommon side effect of spinal or epidural injection is nerve injury.
shorter time to recover
An easier postpartum recovery may be an advantage for women who choose an unmedicated delivery. Although every labor develops differently, some women discover they feel a lot better after an unmedicated birth than a medicated one. The effect of natural hormones is one explanation for this.
A local anesthetic (a painkiller) and steroid medication (Kenalog or de Pomerol) are injected into the epidural space as an epidural steroid injection. Just outside the membrane that protects the spinal cord and nerve roots is where the epidural space is found in the spine.
An uncommon side effect of spinal or epidural injections is nerve injury. Usually, nerve injury is transient. It is extremely uncommon for permanent nerve injury to cause paralysis (loss of function of one or more limbs).
After the epidural drug is removed, the symptoms typically subside after two hours. You could have some cramping and vaginal pain from labor after the effects of the epidural wear off. The area where the epidural was inserted into your back may be tender to the touch and have a slight bruise.