Addison’s Disease
Table of Contents
What is an Addison’s Disease?
Addison’s disease is a chronic condition in which the adrenal glands do not produce enough of the hormones cortisol and aldosterone.
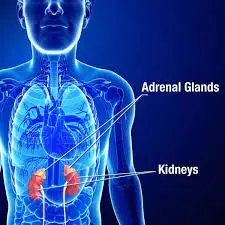
The adrenal glands, also called suprarenal glands are small, triangle-shaped glands that are located on top of each of the two kidneys. They are a function of the endocrine system.
Cortisol is a hormone that helps the body respond to stress, including the stress of illness, injury, or surgery. It also helps maintain blood pressure, heart function, immune system, and blood glucose (sugar) levels. Cortisol is essential for life.
Aldosterone is a hormone that impacts the balance of sodium (salt) and potassium in the blood. This in turn controls the amount of fluid that kidneys remove as urine (pee), which affects blood volume and blood pressure.
Addison’s disease is also called primary adrenal insufficiency. A related disorder, secondary adrenal insufficiency, happens when the pituitary gland does not release enough adrenocorticotropic hormone (ACTH), which activates the adrenal glands to produce cortisol.
Addison’s disease is also called primary adrenal insufficiency. It is an infrequent long-term endocrine disorder characterized by insufficient production of the steroid hormones cortisol and aldosterone by the two outer layers of the cells of the adrenal glands (adrenal cortex), causing adrenal insufficiency.
Addison’s disease occurs from problems with the adrenal gland such that not enough of the steroid hormone cortisol and possibly aldosterone are produced. Addison’s disease is an autoimmune disease that affects some genetically prone people in whom the body’s own immune system has started to target the adrenal gland. While it can lead to tuberculosis, in many adult cases it is unclear what has triggered the onset of the disease. Causes can contain certain medications, sepsis, and bleeding into both adrenal glands. Addison’s disease is commonly diagnosed by lab tests such as blood tests, urine tests, and medical imaging (adrenal crises may occur in all forms of adrenal insufficiency)
Causes of Addison’s Disease:
The adrenal glands are the small organs that release hormones located on top of each kidney. They are made up of an outer portion, named the cortex, and an inner portion named the medulla.
The cortex produces 3 hormones:
- Glucocorticoid Hormones: Hormones such as cortisol maintain sugar (Glucose) control, lower (suppress) immune response, and help the body respond to stress.
- Mineralocorticoid Hormone: Hormones such as Aldosterone control sodium, Water, and Potassium balance.
- Sex hormones: Androgens (male), and estrogen (female) influence sexual development and sex drive.
Addison’s disease outcomes from the destruction of the adrenal cortex. The damage causes the cortex to make hormone levels that are too less.
This damage may be caused by the following reasons given below:
- The immune system mistakenly attacks the adrenal glands (autoimmune disease)
- Infections such as Tuberculosis, HIV, Fungal infections, etc
- Hemorrhage occurs in the adrenal glands
- Tumors
Classification of Addison’s Disease:
There are two main classifications for Addison’s disease:
Primary adrenal insufficiency and Secondary adrenal insufficiency. In order to treat this disease, the doctor will need to find out what is responsible for the disease.
Primary adrenal insufficiency
Primary adrenal insufficiency occurs when the adrenal glands are damaged so severely that they can no longer produce hormones. This type of Addison’s disease is most often caused when the immune system attacks the adrenal glands. This is called an autoimmune disease.
In an autoimmune disease, the body’s immune system misinterprets any organ or area of the body for a virus, bacteria, or another outside invader.
Other causes which lead to primary adrenal insufficiency include:
- Prolonged administration of glucocorticoids (e.g. prednisone)
- Infections in the body
- Cancer and abnormal growths (tumors)
- Certain blood thinners are used to control clotting in the blood
Secondary adrenal insufficiency
Secondary adrenal insufficiency occurs when the pituitary gland located in the brain can’t produce adrenocorticotropic hormone (ACTH). ACTH conveys the adrenal glands when to release hormones.
It is also possible to produce adrenal insufficiency if a person does not take the corticosteroid medications as the doctor prescribes. Corticosteroid medicine help to control chronic health conditions like asthma.
There are also many other reasons for secondary adrenal insufficiency, including:
- Tumors
- Medications
- Genetics
- Traumatic brain injury
The most common reason for Addison’s disease is an autoimmune response, which occurs when the immune system attacks healthy tissues for an unspecified reason. With Addison’s disease, the immune system attacks the outer portion of the adrenal glands (the adrenal cortex), where they make cortisol and aldosterone. Symptoms do not generally develop until 90% of the adrenal cortex has been vandalized, which can take several months to years.
Approximately 75% of cases of Addison’s disease occur due to an autoimmune attack. Autoimmune Addison’s disease may occur by itself or as part of an infrequent, inherited syndrome, specifically autoimmune polyendocrine syndromes I (APS type-1) and II (Schmidt syndrome).
In history, tuberculosis was the main cause of Addison’s disease. It remains a major cause of the condition in developing countries.
Other less expected causes of Addison’s disease include:
- Reprised infections, including HIV/AIDS-related infections and fungal infections.
- When cancer cells from another part of the body invade the adrenal glands.
- Bleeding (hemorrhaging) into your adrenal glands.
- Surgical removal of your adrenal glands.
- Amyloidosis (A condition in which amyloid proteins build up in vital organs, which can cause damage to the organ).
Mechanisms :
The negative feedback loop for glucocorticoids
Causes of adrenal insufficiency can be classified by the mechanism through which they cause the adrenal glands to make insufficient cortisol. This can occur due to the destruction of the adrenal cortex. These defects include Glucocorticoid and Mineralocorticoid hormones as well. These are Adrenal Dysgenesis (the gland has not formed adequately during the development), Impaired Steroidogenesis (the gland is present but is biochemically not able to produce cortisol), or Adrenal Destruction (disease processes leading to glandular damage).
Adrenal destruction
Autoimmune adrenalitis is the most typical cause of Addison’s disease in the industrialized world. Autoimmune destruction of the adrenal cortex is induced by an immune reaction against the enzyme 21-hydroxylase (a phenomenon first described in 1992). This may be separated or in the context of the autoimmune polyendocrine syndrome (APS type 1 or 2), in which other hormone-producing organs, such as the thyroid and pancreas may also be affected.
Adrenal destruction is also a characteristic of adrenoleukodystrophy, and when the adrenal glands are implicated in metastasis (seeding of cancer cells from elsewhere in the body, especially in the lung), Hemorrhage (e.g., in Waterhouse–Friderichsen syndrome or antiphospholipid syndrome), particular Infections (tuberculosis, histoplasmosis, coccidioidomycosis), or the precipitate of abnormal protein in amyloidosis.
Adrenal dysgenesis
All reasons in this category are genetic, and generally very infrequent. These contain mutations to the SF1 transcription factor, congenital adrenal hypoplasia due to DAX-1 gene mutations, and mutations to the ACTH receptor gene (or related genes, such as in the Triple-A or Allgrove syndrome). DAX-1 mutations may mass in a syndrome with glycerol kinase shortage with a number of other symptoms when DAX-1 is deleted together with a number of other genes.
Impaired steroidogenesis
To make cortisol, the adrenal gland mandates cholesterol, which is then converted biochemically into steroid hormones. Interruptions in the delivery of cholesterol contain Smith–Lemli–Opitz syndrome and abetalipoproteinemia. Of the synthesis issues, congenital adrenal hyperplasia is the most common (in various forms: 21-hydroxylase, 17α-hydroxylase, 11β-hydroxylase and 3β-hydroxysteroid dehydrogenase), lipoid CAH due to shortage of StAR and mitochondrial DNA mutations. Some medications interrupt with steroid synthesis enzymes (e.g., ketoconazole), while others increase the normal breakdown of hormones by the liver.
Symptoms of Addison’s Disease:
What are the symptoms of Addison’s disease?
With Addison’s disease, the damage to the adrenal glands usually happens slowly over time, so symptoms occur slowly. Symptoms differ from person to person.
Symptoms of Addison’s disease include:
- Steadily aggravating fatigue (most common symptom).
- Patches of dark skin are called hyperpigmentation, especially around scars and skin creases and on the gums.
- Muscle weakness
- Abdominal pain.
- Nausea and vomiting.
- Diarrhea.
- Loss of appetite and unintentional weight loss
- Pain in muscle, muscle spasms, and/or joint pain.
- Dehydration due to low sodium.
- Low blood pressure, can cause lightheadedness or dizziness upon standing.
- Changes in mood and behavior, such as irritability, depression, and poor concentration in the patient.
- Lightheadedness upon standing
- A desire for salty food.
- Decreased blood sugar (hypoglycemia).
- People assigned female at birth with Addison’s disease may also have abnormal menstruation (periods), lose body hair, and have a lowered sexual drive.
Some people have desires for salty foods due to the loss of sodium passes in their urine. Hyperpigmentation of the skin may be detected, particularly when the person lives in a sunny region, as well as darkening of the palmar crease, sites of friction, recent scars, the border of the lips, and genital area skin. These skin changes are not engaged in secondary and tertiary hypoadrenalism.
Skin changes:
- Hyperpigmentation of the skin
- Vitiligo
Gastrointestinal changes:
- Nausea and vomiting
- Weight loss
- Abdominal pain
Slight common but still occurring:
- Malnutrition and Muscle wasting
Behavioral disorders:
- Anxiety
- Depression
- Irritability
- Poor concentration
Changes in females:
- Loss or irregularity of the menstrual cycle
- Loss of body hair
- Decreased sexual drive
While physical examination, the following clinical signs may be witnessed:
- Low blood pressure with or without orthostatic hypotension (blood pressure that falls withstanding)
- Darkening (hyperpigmentation) of the skin, including areas that are not exposed to the sun – characteristic sites of darkening are skin creases (e.g., of the hands), nipple, and the interior of the cheek (buccal mucosa); also, an old scar may get darken. This occurs because melanocyte-stimulating hormone (MSH) and ACTH transmit the same precursor molecule, pro-opiomelanocortin (POMC). After the production of hormones in the anterior pituitary gland, POMC gets cleaved into gamma-MSH, ACTH, and beta-lipotropin. The subunit ACTH undergoes further division to produce alpha-MSH, the most important MSH for skin pigmentation. In secondary and tertiary forms of adrenal deficiency, skin darkening does not occur, as ACTH is not overproduced.
- Addison’s disease is associated with the consequence of other autoimmune diseases, such as type I diabetes, thyroid disease (Hashimoto’s thyroiditis), celiac disease, or vitiligo. Addison’s disease may be the only manifestation of not diagnosed celiac disease. Both diseases transmit the same genetic risk factors (HLA-DQ2 and HLA-DQ8 haplotypes).
The existence of Addison’s in addition to Mucocutaneous candidiasis, Hypoparathyroidism, or both, is called autoimmune polyendocrine syndrome Type1. The presence of Addison’s in addition to autoimmune thyroid disease, type 1 diabetes, or both, is called autoimmune polyendocrine syndrome Type2.
In some cases, such as after an injury or extreme illness, or time of intense stress, symptoms can arrive quickly and cause a life-threatening event called an Addisonian crisis or Acute Adrenal Failure.
An “Adrenal crisis” or “Addisonian crisis” is a constellation of symptoms that characterizes intense adrenal insufficiency. This may be the consequence of either previously not diagnosed Addison’s disease. A disease approach suddenly affecting the adrenal function (such as adrenal hemorrhage), or an intercurrent problem (e.g., infection, trauma) in someone known to have Addison’s disease. It is a medical emergency and potentially a life-threatening condition requiring immediate emergency treatment.
An Addisonian crisis is a medical emergency. If it is not treated, it can conduct to shock and death. Manifestation of an addisonian crisis includes:
- Extreme weakness.
- Sudden, severe pain in the lower back, belly, or legs.
- Feeling agitated, confused, afraid, or other mental changes.
- Severe vomiting and diarrhea, potentially directing to dehydration.
- Low blood pressure.
- Loss of consciousness.
- Impulsive penetrating pain in the legs, lower back, or abdomen
- Severe vomiting and diarrhea, resulting in dehydration
- Low blood pressure
- Syncope (Loss of consciousness and loss of ability to stand)
- Hypoglycemia (reduced level of blood glucose)
- Confusion, psychosis, slurred speech
- Extreme lethargy
- Hyponatremia (low sodium level in the blood)
- Hyperkalemia (elevated potassium level in the blood)
- Hypercalcemia (elevated calcium level in the blood)
- Convulsions
- Fever
Contact the nearest hospital as soon as possible if a person has these symptoms.
Risk factors:
Addison’s disease affects:
Addison’s disease can affect people of any age group, but it is most common in people 30 to 50 years old.
People who have Autoimmune Polyendocrine Syndrome, an infrequent, inherited condition in which the immune system wrongly attacks many of the tissues and organs, are much more likely to have Addison’s disease. Mucous membranes, adrenal glands, and parathyroid glands are commonly affected by this syndrome, though they can affect other types of tissues and organs.
People who have the following autoimmune disease are also at more increased risk of developing the autoimmune (most common) form of Addison’s disease:
- Type I diabetes
- Pernicious anemia
- Graves’ disease
- Chronic thyroiditis
- Dermatitis herpetiformis
- Vitiligo
- Myasthenia gravis
Diagnosis:
The diagnosis is confirmed by demonstrating low cortisol and aldosterone levels, high renin level, and a blunt cortisol response with ACTH stimulation. The approach for the evaluations is as follows;
An inappropriately low cortisol level.
Evaluating the adrenal cortex’s functional capacity to synthesize cortisol and determine whether the cortisol deficiency is related to a corticotropin (ACTH) deficiency. This will help to classify whether adrenal insufficiency is primary or secondary.
Determining whether a treatable reason is present. Further evaluation should be done to determine its underlying cause.
Cortisol Level
Typically, a lower random cortisol level is seen. Cortisol follows a diurnal pattern, in which it is highest in the early morning; therefore, an early morning level should be obtained. However, it is not practical to acquire an early morning cortisol level in the emergency department (ED). Also, these results are not readily available at the time of the ED presentation. A single determination of serum cortisol level is not adequate to assess adrenal function. However, a morning cortisol level above 18 micrograms/dL is considered normal and may exclude the diagnosis. A low cortisol level (< 3 mcg/dL) is adequate to diagnose adrenal insufficiency.
18 microgram/dL = Normal
<3 microgram/dL = Adrenal insufficiency
3-19 microgram/dL /dL = Equivocal and further evaluation is suggested.
ACTH Level and Corticotropin Stimulation Test
The ACTH level is markedly increased in primary adrenal insufficiency. However, the level is not elevated or within the reference range in patients with central adrenal insufficiency. In equivocal cases, the diagnosis is confirmed by an ACTH (cosyntropin) stimulation test. It causes rapid stimulation of cortisol and aldosterone secretion. This is a first-line diagnostic test for the evaluation of adrenal insufficiency. Plasma cortisol levels should be measured at 0 and 30 to 60 minutes after administration of ACTH. CRH stimulates ACTH release from the pituitary. In primary adrenal insufficiency, a high ACTH level is present, which rises further after CRH stimulation but is not able to stimulate serum cortisol secretion. However, in secondary adrenal insufficiency, a low ACTH level is seen, failing to respond to CRH.
Primary adrenal insufficiency: elevated ACTH
Central adrenal insufficiency: abnormally normal or low ACTH
With ACTH stimulation
Normal response = Adequate response (peak cortisol level >18 mcg/dL)
Adrenal insufficiency = Less or no response
Aldosterone and Renin Level
Serum renin and aldosterone levels should be brought to determine whether a mineralocorticoid deficiency is present. Both cortisol and aldosterone are not present in primary adrenal insufficiency. A lower aldosterone concentration is present despite markedly decreased plasma renin activity. In secondary adrenal shortage, the aldosterone level will be regular. Increased plasma renin activity can be seen; this suggests that there is adrenal cortex dysfunction. A high level happens when there is a low level of serum aldosterone.
Serum Chemistry
Typical laboratory findings contain hyponatremia, hyperkalemia, and hypoglycemia.
- Hyponatremia is due to cortisol and aldosterone deficiency. Aldosterone deficiency induces sodium wasting, and cortisol deficiency results in an increased antidiuretic hormone. This causes raised water absorption.
- Hypovolemia also starts ADH secretion.
- Hyperkalemia is secondary to reduce aldosterone levels, which causes natriuresis and potassium retention. Hyperkalemia does not occur in secondary disease; this helps to determine it from primary adrenal insufficiency.
- Hypoglycemia is multifactorial, including reduced oral intake and lack of glucocorticoids, which are necessary for gluconeogenesis. Hypoglycemia is multifactorial, including diminished oral intake and lack of glucocorticoids, which are necessary for gluconeogenesis.
- Hypercalcemia may be present, which mirrors extracellular fluid loss.
TSH Level
A slight elevation of TSH level can happen in adrenal insufficiency. This occurs due to a reduction in cortisol levels and abnormal TSH circadian rhythm. If TSH rises continue, the possibility of hypothyroidism should be considered.
Anti–21-hydroxylase Antibodies
Anti-adrenal antibodies (such as 21-hydroxylase antibodies) are markers of autoimmune devastation of the adrenal gland. The 21-hydroxylase enzyme is demanded cortisol synthesis in the adrenal cortex. This may be useful to determine the cause of Addison’s disease. It is also crucial to evaluate other organ-specific autoimmune conditions.
Routine laboratory investigations may reveal:
- Lower blood sugar (worse in children due to loss of glucocorticoid’s glucogenic effects)
- Lower blood sodium, due to loss of production of the hormone aldosterone, the kidney is not the ability to excrete free water in the absence of sufficient cortisol, and also the effect of corticotropin-releasing hormone to stimulate secretion of ADH.
- High blood potassium, due to loss of production of the hormone aldosterone.
- Eosinophilia and lymphocytosis (increase in the number of eosinophils or lymphocytes, which are two types of white blood cells)
- Metabolic acidosis (increased blood acidity) is also due to loss of the aldosterone hormone because sodium reabsorption in the distal tubule is linked with acid/hydrogen ion (H+) secretion. Absent or insufficient levels of aldosterone stimulation of the renal distal tubule lead to sodium wasting in the urine and H+ retention in the serum.
Additional Studies
Additional studies should be done to determine the underlying cause. A PPD test should be conducted to evaluate for tuberculosis. Plasma’s very long-chain fatty acid profile should be obtained in cases where adrenal leukodystrophy is suspected. A complete blood count (CBC) may show neutropenia, lymphocytosis, and eosinophilia. ECG may indicate tall and peaked T waves if hyperkalemia is present while testing. Histology is valuable to investigate infiltrative causes of adrenal insufficiency. The finding of caseating granulomas may indicate tuberculosis, whereas a non-caseating granuloma may be due to sarcoidosis
Imaging Studies:
A chest radiograph may indicate a small heart; this may be due to a reduction in the cardiac workload. In doubted cases of adrenal hemorrhage, an Abdominal CT scan may be done to provide useful information in determining the cause of the disease. For example, bilateral overgrowth of the adrenal glands may be seen with adrenal hemorrhage.
Computed tomography (CT scan): Computed tomography is an imaging test that utilizes computers to combine many X-ray images into cross-sectional views. Your doctor r may order a CT scan to evaluate your adrenal glands and pituitary gland. For example, it can show if the immune system has damaged the adrenal glands or if the glands get infected.
Autoimmune adrenal destruction– i.e., Small adrenals.
Tuberculosis– i.e., Calcification or hemorrhage.
A magnetic resonance imaging (MRI) of the hypothalamic-pituitary region should be accepted if ACTH is inappropriately low in the presence of cortisol deficiency. A pituitary baseline profile should also be acquired. The radiographic findings are non-specific. A biochemical diagnosis of adrenal insufficiency should be done before getting radiographic examinations.
Complications:
Addisonian crisis. If a person has untreated Addison’s disease, they may develop an Addisonian crisis as a result of stress on the body, such as injury, infection, or illness. Normally, the adrenal glands make two or three times the normal amount of cortisol in response to physical stress. With adrenal insufficiency, not being able to increase the quantity of cortisol made as a result of stress can lead to an Addisonian crisis.
An Addisonian crisis is a life-threatening condition that results in low blood pressure (Low BP), low blood levels of sugar, and increase blood levels of potassium. It needs immediate medical care.
Other autoimmune diseases. People with Addison’s disease frequently have related autoimmune diseases.
Treatment of Addison’s Disease:
Early recognition is necessary for the management of adrenal insufficiency. Addison’s crisis is an extreme endocrine emergency; immediate identification and treatment are required. Be aware that if it is not recognized and treated, the adrenal crisis can be fatal. The confirmatory laboratory diagnosis should not delay the treatment. Blood samples should be checked for subsequent measurement of ACTH and cortisol levels. The elevation of ACTH with less cortisol is diagnostic of primary adrenal deficit. Measurement of cortisol in the ACTH stimulation test may be achieved when initial laboratory evaluation cannot establish the diagnosis. Plasma renin level is often increased and it is indicative of a mineralocorticoid deficiency, accompanied by a low aldosterone level.
Acute Phase
Patients with an adrenal crisis need the following:
- Fluid resuscitation to restore the intravascular volume with intravenous (IV) normal saline.
- Dextrose to correct hypoglycemia.
- Modification of the hormone deficiency, both glucocorticoid, and mineralocorticoid
- The primary hormonal treatment is the management of hydrocortisone. The initial dose for Adrenal crisis is 100 mg IV bolus, followed by 50 to 100 mg IV every 6 hours over 24 hours. In children, the dose is 50 mg/m^2 (max: 100 mg) IV bolus, followed by 50 to 100 mg/m^2. Since this dose has important mineralocorticoid activity, mineralocorticoids such as fludrocortisone are unneeded during the acute phase. Dexamethasone 4-mg IV boluses can be regarded in the ED when emergent steroid administration is required; it is less likely to interfere with the serum cortisol assays. It is long-acting and does not hinder biochemical assays of endogenous glucocorticoid production. Prednisone and dexamethasone have small or no mineralocorticoid activity. The initial fluid replacement is a normal saline bolus followed by 5% glucose in isotonic saline. Hypoglycemia should be treated promptly.
Maintenance Phase
Life-long treatment with hormonal replacement is needed. The maintenance therapy goal is to provide a replacement to maintain physiologic glucocorticoid and mineralocorticoid levels. The standard doses are as follows;
- Glucocorticoid: Hydrocortisone 5 to 25 mg/day (Can be divided into 2 or 3 doses)
- Prednisone 3 to 5 mg/day
Doses should be changed according to the clinical reaction and normalization of electrolyte abnormalities. To reduce adverse effects, the dose should be titrated to the lowest possible dose that controls the symptoms; ensure the patient is clinically okay. Plasma Renin levels can also be manipulated to adjust the doses. Serum ACTH levels may change significantly and cannot be used for dose adjustment. It is essential to consider concurrent medications when deciding the glucocorticoid dose. For Example, certain drugs such as Rifampin can improve hepatic glucocorticoid metabolism and may inactivate cortisol. Dexamethasone is not a suitable choice for maintenance treatment; the dose titration is difficult and increases the risk of the Cushing effect.
- Mineralocorticoid: Fludrocortisone 0.05 to 0.2 mg daily.
- In children, the initial hydrocortisone drug dose is 8 mg/m^2/day orally; this dose can be divided into 3 or 4 doses.
Fludrocortisone should be given at an acceptable dose to keep the plasma renin level in the reference range. A raised PRA indicates a more increased dose of fludrocortisone is needed. The mineralocorticoid dosage should be checked to address the degree of stress. Similarly, the identification and treatment of underlying causes such as sepsis are required for an optimal result. The treatment of associated conditions should also be supplied.
Treatment Considerations:
- In patients with Addison’s disease, glucocorticoid secretion does not rise during stress. Therefore, in the existence of fever, infection, or other illnesses, the hydrocortisone dose should be raised to compensate for a possible stress response.
- In general, a standard stress dose is 2-3 times the daily maintenance dose.
- Patients taking rifampin need an increased dose of hydrocortisone, as it enriches the clearance of hydrocortisone.
- The thyroid hormone can boost the hepatic clearance of cortisol; this can precipitate an adrenal crisis. Glucocorticoid replacement can potentially normalize thyroid-stimulating hormones.
- In patients with concomitant diabetes insipidus, glucocorticoid therapy can worsen diabetes insipidus. Cortisol is needed for free-water clearance, and cortisol deficiency may prevent polyuria.
- Pregnancy, particularly during the third trimester, raises corticosteroid requirements.
Outcomes:
The prognosis for Addison’s disease is typically good. Although people who have Addison’s disease will require to take medicine for the rest of their lives, they can live normal, healthy lives.
Addison’s disease is a life-threatening condition that needs accurate diagnosis and prompt treatment. If the diagnosis is delayed, it holds high morbidity and mortality. Thus, the condition is best handled by an interprofessional team of healthcare workers that includes an endocrinologist, an intensivist, an infectious disease specialist, and a pharmacist. Education of the patient is essential for patients with chronic conditions. Physicians, nurses, and pharmacists can intercommunicate this responsibility.
Nurses need to assist with treatments, monitor patients, and provide updates to the team. Once the diagnosis is confirmed, the outcomes depend on the primary cause. Any delay in initiating the treatment can direct to poor outcomes. All patients who have Addison’s disease must be urged to wear a medical alert bracelet. Patients should be educated about the signs and symptoms of the disease. They should be encouraged to contact their primary care provider if there see any warning symptoms. Ultimately, in times of stress, such as fever, patients should be instructed to double the dose of steroids and see their primary care provider.
The dose of these medications, however, needs to be closely monitored to prevent over- or under-treatment. Over-treatment with glucocorticoids (hydrocortisone) will result in obesity, Type 2 diabetes, and osteoporosis. Over-treatment with fludrocortisone can induce high blood pressure (hypertension).
Up to 50% of people with Addison’s disease develop another autoimmune disease.
FAQs:
How is Addison’s disease cured?
This disease is treated with medicine to replace the missing hormones. A person needs to take the medication for the rest of her life.
What is the treatment for an adrenal crisis?
Treatment. In an adrenal crisis, an intravenous or intramuscular injection of hydrocortisone (an injectable corticosteroid) must be given instantly. Supportive treatment of low blood pressure with intravenous fluids is usually required. Hospitalization is mandated for adequate treatment and monitoring.
How is Addison’s disease diagnosed?
If Addison’s disease is imagined, blood tests will be carried out to measure the levels of sodium, potassium, and cortisol in your body. A low sodium, high potassium, or low cortisol level may indicate Addison’s disease
What is the best vitamin for adrenal glands?
B vitamins such as B1 (thiamine), B5 (pantethine), and B12 all directly affect the adrenal glands’ cortisol response to stress. Vitamin B3 (niacin) and B12 also play a role in the sleep/wake cycle which can be affected by stress and cortisol.
Is walking good for adrenal fatigue?
Solutions for restoring adrenal function: Perform simple, low-intensity motion. Get up and go for a walk for a half hour in the morning—just get low-level general activity. This will assist with mental performance too.


2 Comments