A Post vaccination symptoms in health care workers
Table of Contents
ABSTRACT
BACKGROUND
Though India has launched the corona virus vaccination drive, any data proving the vaccine s efficacy as well as post vaccination side effects has not been published in the public domain, including any results of an interim analysis
METHOD:
A Descriptive Survey was conducted from 10/09/2020 to 25/10/2020 using convenient random Sampling Technique. To assess post vaccination symptoms among the beneficiaries of COVID vaccine, a questionnaire was prepared on Google Form and was share among all the health professionals who received the first dose of vaccine to willingly fill the form.Total 100 beneficiaries responded for the same.
RESULT:
Majority of healthcare professionals who completed the survey reported mild and short-lived post-vaccination symptoms. Fever, chills, tiredness, mayalgia and headache were most commonly reported. No serious events were reported. Majority of the beneficiaries stated that the post vaccination symptoms started appearing after 8-10 hours of vaccination which affected their next day work
CONCLUSION
Greater awareness and anticipation of adverse effects among healthcare workers may help to spread public awareness through their own experiences of COVID Vaccination during next phase
KEYWORDS
Post Vaccination Symptoms, COVID vaccine.
INTRODUCTION
Corona virus is called as covid-19 which is associate with breathing problems. The virus was first reported from Wuhan city in china in the end of 2019, which in less than 3 month spread throughout the world.A human corona virus first identified by the scientist in 1965. It caused a common fever, breathlessness, cough, sore throat.Then declared a global pandemic by the WHO on March 2020 .ongoing pandemic severely damage world’s most developed country like USA, RUSSIA E.T.C and becoming a major threat for low and middle income nation.However the number of cases continues to rise throughout the world and became serious menace to public health. This virus invalids are throat, respiratory tract and lungs and starts to deactivate very quickly.The spread of the virus is more dangerous because it is transmitted from person to person.In addition, transmission can be carried out without problems in the “3 CS (where the risk of spreading COVID -19 is higher where these “3 CS” overlap)
1- Closely connected environment ( Crowded places with many people nearby )
2- Close – contact settings, people stand too close with each other
The spread can be because of air, surface the person infected with this virus has the feeling of being infected when it enters the human body, although there has been a steady increase in the recovery rate by the steroids.In starting of 2021 India launched the vaccine, which was the biggest vaccination happened across the world. First priority was given to the front line workers , medical student,medical staff and defense employees, In order to avoid on the last moment by command of Ruling Government had issued detailed guidelines on mandatory precautions need to be precise by thos who managing The vaccines. The second part of the vaccination drive is the association of community. The direction of achieving the “HERD IMMUNITY” in The Nation As a part of the campaign three vaccines were introduced
THEY WERE RESPECTIVELY
1) COVISHIELD
2) COVAXIN
3) PFIZER
Are being given to the people
The commanding companies who made the vaccines:
1) BHARAT BIOTECH made COVAXIN in collaboration with the ICMR and NIV for the treatment of covid-19. In starting of 2020 BHARAT BIOTECH was contracted by precis medicament for the supplies of co-vaccine to Brazil.
2) Astra zeneca covid-19 –AZD122 vaccine is marketed as Covishield , developed by the university of oxford and manufactured in India by the pune Serum Institute of India.Astra Zeneca covid-19 –AZD122 vaccine is marketed as covishield, developed by the University of Oxford and manufactured in India by the Pune Serum Institute of India.This is a Vector virus vaccine based primarily on a lack of replication of the ad rhinovirus (a weakened model of the common cold virus) which causes cold in the CHIMPANZEES but it cannot effect the humans. It attaches itself to cells and requires them to inject DNA to create mutant Corona virus proteins. This shape is called the corona virus layer and produces nodules. It is determined to be an alien and is interested in the immune system, which can resist the actual Coronavirus at the time of the actual infection. The advantage of the vaccine that it’s cheaper & important. It causes be Transported, Stored & Settled at Standard Refrigerator Temperatures.The Indian indigenous vaccine by BHARAT Bio Techs developed jointly with the Medical Research council of India (ICMR), the Natural Visso foundation laboratory (NIV).
Indian inactivation vaccines are developed and manufactured in Bharat BiotechBSL-3 (Bio Security level 3) high level installations.
Vaccines are being developed using platform techniques derived from Vero Verion Cells.Inactivation vaccines are not replicated and, therefore, are low in pathological effects a low chance to cause. They contain dead viruses and cannot infect people, but the immune system can join a protective response to infection.
NEED OF STUDY
Vaccination drive for the coronavirus is the widest drive occurring globally vaccination drive was initially launched by INDIA.There is no study relevant to the post COVID-19 vaccination symptoms & the Vaccine’s efficacy.So the study was conducted for the post covid vaccination symptoms in younger population
AIMS AND OBJECTIVES
AIMS OF STUDY-
Main aim of the study is to find out the prevalence of spectrum Of vaccination symptoms profile in the younger individual.
OBJECTIVE OF STUDY-
To identify the suffering & severity of the symptoms of post covid vaccination in Indian population
REVIEW OF LITERATURE
- Nag-la a elShitary did a study on “ Minor to moderate side effects of Pfizer Biotech Covid 19 Vaccine amount Saudi resident. Retrospective cross sectional study. The study concluded that most of the side effect reported were very consistent with this sheet for recipients and care given.
- S.AMeo , and all studied on Covid 19 vaccine comparison of biological pharmacological characteristic and adverse effect of Pfizer and Moderna vaccine & the result of study conclude that both the vaccines were beneficial to fight against SARS Cov-2, infection and improving0 immunity.
- Kamal K Chemali and all, studied on the post Covid 19 chronic symptoms and we conclude that the study includes symptoms which are followed by fatigue subjective ,fever, headache and body pain, symptoms of autonomic impairment.
- Kathrym J. Gray MD (2020) had a research study on ‘ CORONAVIRUS’ disease 2019 vaccine response in pregnant & lactating woman ; A short study Total of 131 reproductive age vaccine recipients were collected for the research purpose and the result showed positive response
that is in vaccine induced antibody tiers were equivalent in pregnant & lactating woman when composed with non – pregnant woman. - Ronald N.Kostoff,in September, 2021 studied on covid-19 vaccine safety and the study concludes that there is no substitute currently available for long term human clinical trials to ensure long-term human safety.
- Michelle Ngirbabul in March 2021 studied on covid-19 vaccine acceptance among pregnant woman and mothers of young children results of survey in 16 countries. The study concluded that the covid-19 vaccine acceptance in pregnant woman and mother of young children and its
predictors among woman vary globally. - Riccardo levi, did a study in one dose of SARS – COV2 vaccine exponentially increases antibodies in recovered individuals with symptomatic COVID 19 in 2021. Total of 124 healthcare professionals with or without symptoms were included in the study. The study concluded that
one dose of vaccine is sufficient in symptomatic SARS – COV2 exposed subjects and it also suggests that no need for second dose, particularly in light of current vaccine shortage
MATERIALS AND METHODOLOGY
Methods of collection of data
• Study design : Observational study
• sample size : 100
• sample design : Random sampling
• source of data : AHMADABAD
• duration of study : 45 days
MATERIALS USED FOR THE STUDY
• Consent form
• Mobile phone
• Google form
• Pen/Pencil
INCLUSION AND EXCLUSION CRITERIA
INCLUSION CRITERIA
• 18 to 32 years
• 100 young people
• Gender – both male and females
• Language – English
EXCLUSION CRITERIA:-
• Not vaccinated
• Above 32 years
• comorbidity
STATISTICAL ANALYSIS
Descriptive statistics were performed for the collected data the responses were displayed as a frequency.
RESULT
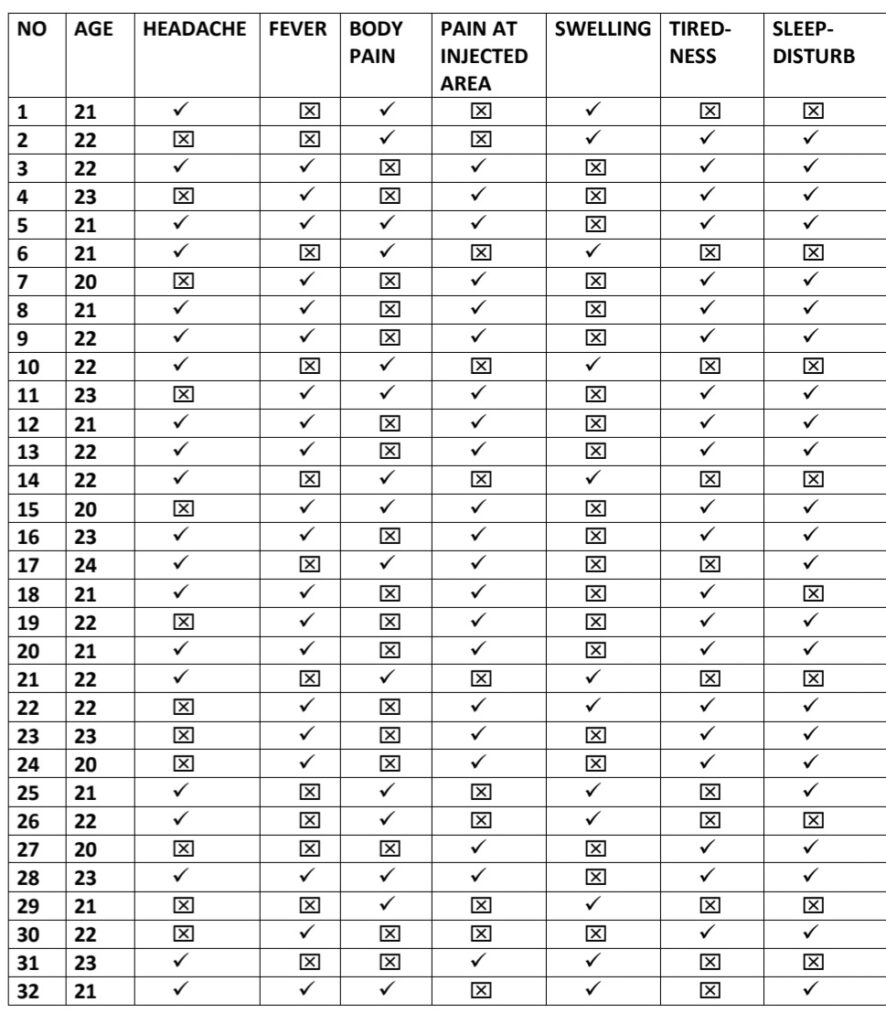
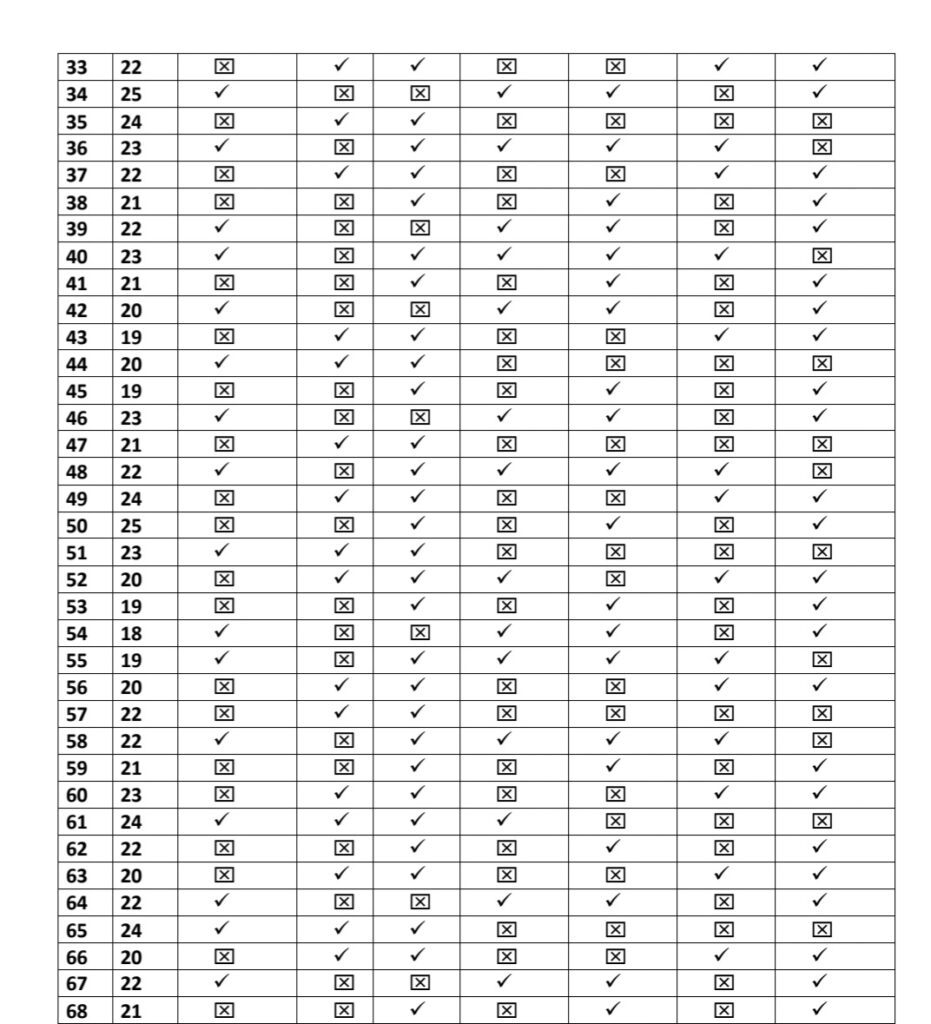
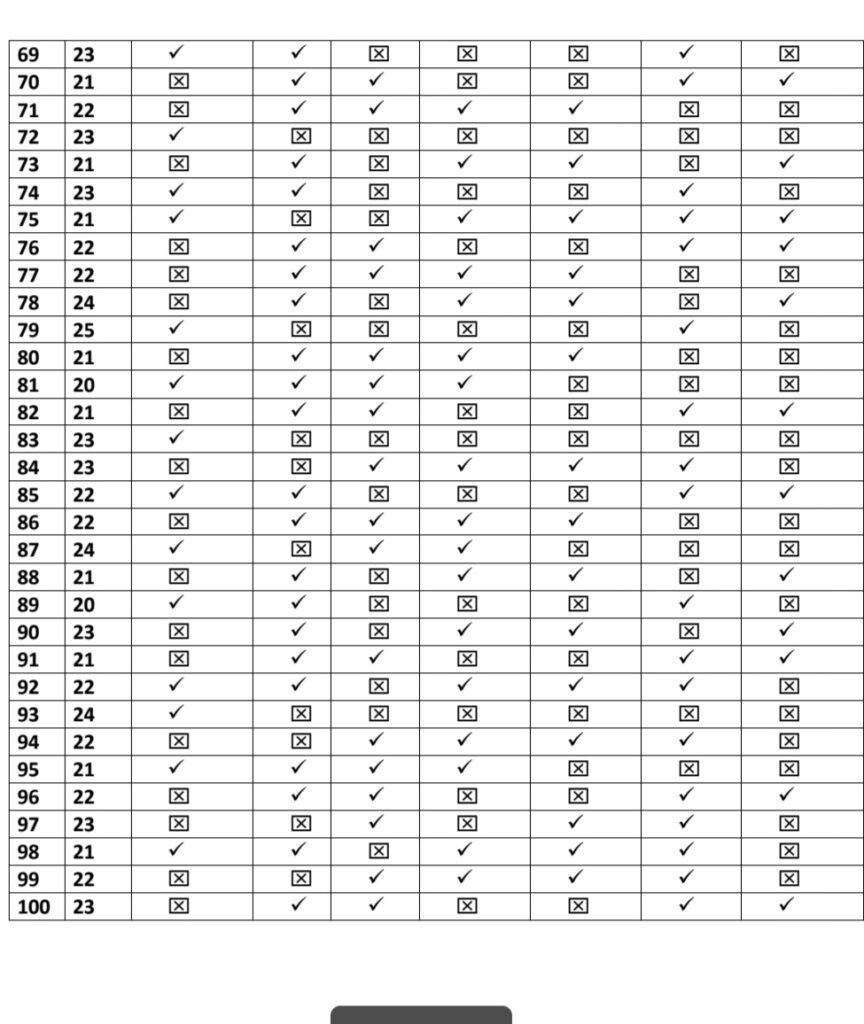
- We Selected 100 people for our thesis which is only 8% of total healthcare workers
- Every people replied affirmatively the survey and accepted for the same which is based
on their vaccination symptoms. - We have just calculated young age population for our research
- Among this 91% TAKEN COVISHILD and 8% taken COVAXIN and 1% take PFIZER
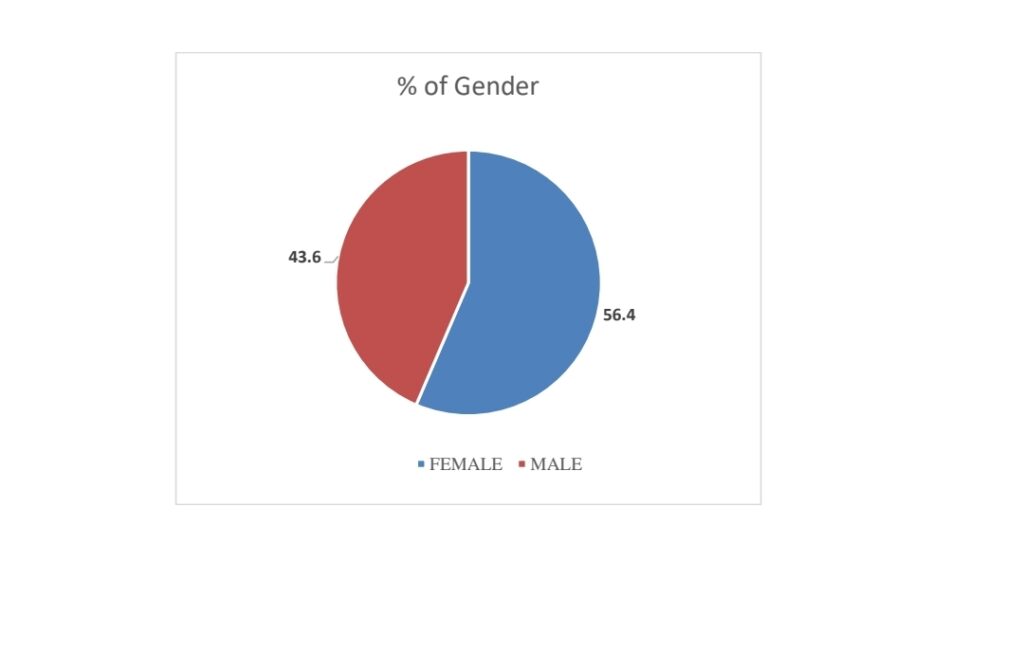
1.GENDER:
The thesis shows that females are more likely elected than males.
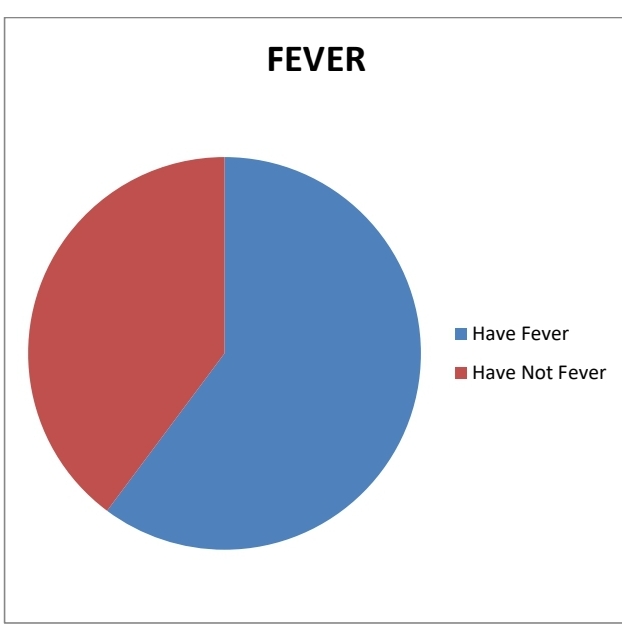
2.FEVER:
The majority of the people were suffered from fever only 39% of people didn’t
have any fever symptoms.
- MEDICINES TAKEN FOR THE FEVER:
Research shows that 65% of the
people had taken PARACETAMOL and 23% of people had taken DOLO 500. - BODY PAIN:
Bunch of people had problems like body pain and only 40%
people don’t had any body pain and majority of the people didn’t take any type
of medicines. - HEADACHE:
Half of the people had taken vaccine they suffered from Headache
and 80% of people not taken any type of medicine. - SLEEP DISTURBANCES:
Majority of people have suffered from this
symptoms. - AFFECTED MENSTRUAL CYCLE:
Only 6% of females menstrual cycle
has been affected. - INCONVIENCIES IN WORK:
- Majority of the people have Inconviencies in
work.
METHODOLOGY
The approach for the Research is – Quantitative Research Design. Which is a- Descriptive Survey of Design Research Setting and the study was conducted among the beneficiary of COVID vaccines AHMADABAD Population – All the health care professionals who have received the first dose or both dose of COVID vaccine (Covishield, Covaxin, Pfizer biotech) and have crossed three days following vaccination.Data Collection – Data collection for study was done from 15/03/2021 to 12/04/2021.
To assess post vaccination symptoms among the beneficiaries of COVID vaccine, a questionnaire was prepared on Google Form and was shared with all the health professionals to willingly fill the form & submit. Sample- In the present study, the samples comprised of 100 beneficiaries of COVID vaccine.
Sampling Technique-The present study adopted Non Probability- Convenient Sampling Technique.
Description of Tool- The tool consists of a self-administered questionnaire which includes demographic variables and experience related to COVID-19 vaccination.
DISCUSSION
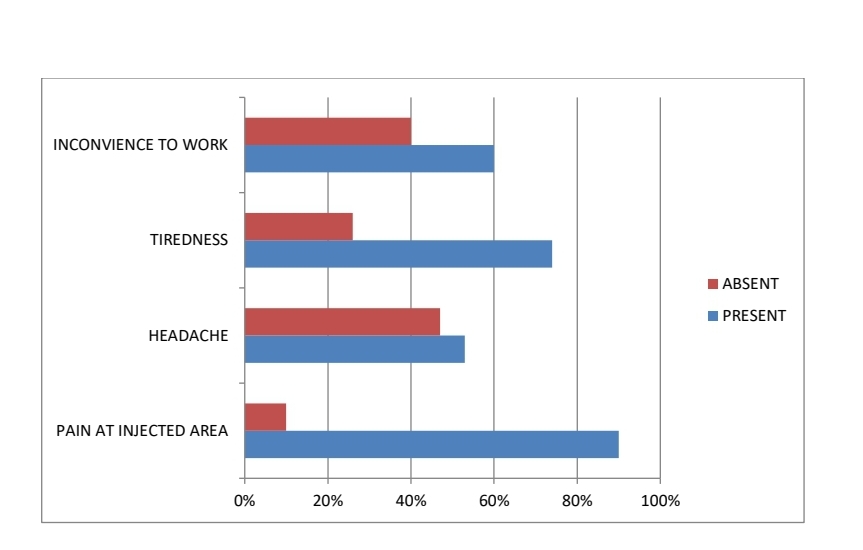
Healthcare professionals who took the survey provided of their immediate post vaccination experience. Two-third of survey respondents reported mild and predictable symptoms following vaccination. The remaining one-third did not report any symptom. TIREDNESS was the most common symptoms (74%), followed by myalgia (44%), FEVER (40%), HEADACHE (48%), SWELLING at injected site (37%), and INCONVENIENCE to work (60%), SLEEP DISTURBANCE (34%), Local pain at injected site was reported by 90% of the respondents.
None of the symptoms were of serious or requiring hospitalization. Symptoms appeared within 12 hours after vaccination, mean duration was 30 hours, 90% reported that post –vaccination symptoms severity was as they has expected or milder. 10% reported that the symptoms were worse enough to affect work the following a day. The frequency of experiencing symptoms following each vaccine were 70%(Covishield), 25 %(Covaxin) , 5% (Pfizer ). There was a clear linear relationship between age and post-vaccination symptoms; this indicates that the retrogenicity of the vaccine decreases with age. In the youngest age group, 85% of people have symptoms, while only 10% of people over 60 have any symptoms. It is known that the reatrogenicity of the vaccine is related to the transient increase of inflammatory cytokines, but it is not considered a reliable indicator of an ideal immune response.
(1)Women area unit additional possible to expertise symptoms when vaccination. during this cluster, the onset of symptoms was a touch earlier and lasted a touch longer. This observation is consistent across all age teams. The findings are dissociated with the results of the immunizing agent trials that are proclaimed. within Astra-Oxford ChAdOx1 nCoV-19 section 2\/3 trial, with a minimum of one general symptom Among 86% of participants rumored when 7% of receiving a customary dose of the immunizing agent In 2010, it accounted for 77%f individuals aged 18 to 21, and 65% aged 25
(2)Placebo injection while discussing vaccination experience similar symptoms appear. In a phase 3 clinical trial of the Pfizer-Biotech vaccine the incidence of post-vaccination headache was temporarily 42% for the vaccine team. People who took saline placebo
(3). This is called the no time signal effect raising expectations for adverse effects of intervention.
(4)These learn about did no longer measure antibody response after vaccination. Therefore can’t after vaccination amongst the elderly, muted signs and symptoms Immune aging. Symptoms are known to be related with antibody neutralization. Copyright holder for this Levels between COVID-19
(5) the presence of post-vaccination symptoms Stable prediction of antibody reaction.
(6) The frequency of use of paracetamol to reduce symptoms after vaccination varies from it is 71% in the age group 20-26 years and 16% in the age group30 years. This is the same as the frequency of symptoms in these subgroups. Although acetaminophen can be accepted to relieve discomfort after vaccination
(7) the routine preventive use of analgesics is not recommended because there is evidence that the immune response is weakened. Fear of what is not known is one of the factors that make people suspicious of vaccines. Explaining the expected results, you can rest assured if you find this information for anyone interested in a new vaccine. In fact, the slight, predictable and short-lived symptoms after vaccination can help reduce vaccination hesitation
CONCLUSION
In general, the side effects from covidshield and covaxin are quite less. There are minimal side effects of vaccine. All the positive findings are reported in this thesis. However I personally have observed all the recipients for a short span of time immediately after receiving the vaccine. So
therefore, as per my findings I conclude that there are no major side effects of these vaccines
SUMMARY
The study is based on the post covid 19 vaccination symptoms in younger population. The study was done to find out the post vaccination symptoms and the side effects of covid 19 vaccine.The vaccines which were included are covishield, covaxin and pfizer. So the study was on the
symptoms after the vaccination and to know about the side effects of the vaccine. Total of 100 subjects were taken who were vaccinated and were aged between 18 to 32 years. A questionnaire was prepared on the google forms and the subjects were asked to fill the form regarding the post
vaccination symptoms.The results show that there was mild symptoms after the vaccination process but no serious symptom was reported and it also shows that there were no side effects of the vaccines
LIMITATIONS AND FURTHER RECOMMENDATIONS
Limitation
Limited age crew – (18years-25years ) was only included.
Selected limited area.
limited pattern size
FURTHER RECOMMENDATION
You may also need to choose a massive area.
Larger pattern measurement.
Teams of all ages have to participate.
Follow-up agreement.
Physical exercise can be added to help complete physical therapy
intervention.
REFERENCES
- Herve, CLaupeze, B, DelGiudice, G. et al. The how’s and what’s of vaccine
reatro genicity. Npj Vaccines 4,39,2019 https://doi.org/10.1038/s41541-019-01232-6 - Safety and immuno genicity of ChAdOx1 nCov-19 vaccine administered in a prime-boost
regimen in young and old adults(COV002): a single-blind, randomized, controlled, phase
2/3 trial Lancet 2020; 396: 1979-93 November 19,2020 - Polack FP et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
December 31,2020. NEngl J Med 2020; 383:2603-2615
DOI: 10.1056/NEJMoa2034577 - CollocaL,Miller FG. The nocebo effect and its relevance for clinical practice.
Psychos-om Med, 2011;733(7);598-603.
Doi:10.1097/PSY.0b013e3182294a50 - Post N et al, Antibody response to SARS-COV-2 infection in humans:A systematic
review Plosone December 31,2020 - Christian LM, Porter K, Carlson E, Schultz-Cherry S. Pro inflammatory cytokine
response correspond with subjective side effects after influenza virus vaccination.
Vaccine.2015;33(29);3360-3366. Doi:10.1016/j.vaccine.2015.05.008 - Jennifer S. Chen, Mia Mandel Alfajaro, Ryan D. Chow, Jin Wei, Renata B. Filler,
Stephanie C Eisenbarth, Craig B Wilen. Non-steroidal anti-inflammatory drugs dampen
the cytokine and antibody response to SARS-COV-2 infection. Journal of Virology Jan
2021, JVI.00014-21;doi 10.1128/JVI.00014-21 - Sale-he, Moody MA, Walter EB. Effect of anti pyre-tic analgesics on immune responses to
vaccination. Hum Vaccine Immune other. 2016;12(9):2391-2402.
Doi10.1080/21645515.2016.1186077 - COVID-19 Vaccines, World Health Organization(internet),(cited 2021 feb 02),
available from: COVID-19 vaccines (who.int) - COVID VACCINE(internet)(cited 2021 Feb 01), Available from:Information
Reading COVID-19 vaccine (mohfw.gov.in) - Li,YD,Chi.,WY.,Su, JH. Et al. Coronavirus vaccine development: from SARS and MERS
to COVID-19. J Bio med Sci 27,104(2020). https://dpo.org/10.1186/s12929-020-00695-2 - Na or Barbarize, Tom Ingres by International vaccine Access Center (NB-Z), and Center for
Health Security (TI), Jones Hopkins Bloomberg School of Public Health, Baltimore, MD
21205, USA. www.thelancet.com Col 396 September 26,2020. - SallamM Dababseh, D,; Eid, H,; AL-Mahzoum, K, Al-Haider, A,; Taim, D,; Yaseen,
A,; Abalone, N,A,; Bakri, F.G,; Mahafzah, A. High Rates of COVID-19 Vaccine Hesitancy
and Its Association with Conspiracy Beliefs: A Study in Jordan and Kuwait among Other Arab
Countries. Vaccines 2021,9,42. http://doi.org/10.3390/vaccines9010042 - Yoda, T,; Katsuyama, H. Willingness to Receive COVID-19 Vaccination in Japan.
Vaccines 2021,9,48. http://doi.org/10.3390/vaccines9010048
ANNEXURE1
concert form
TITLE : POST VACCINATION SYMPTOMS FOR YOUNG
POPULATION.
NAME :
AGE/GENDER:
DATE:
The content of the information provided have been explained in
detail to me, in a language that I comprehend and I have fully understood
and I am willingly participating without any force. The data given by me
will only be used for the research purpose only.
SIGNATURE OF PARTICIPANT:
master chart