Physiology – Blood Clotting Mechanism (Coagulation)
Table of Contents
Introduction
The clotting mechanism, also known as coagulation, is a complex process that occurs in response to tissue damage or injury. The primary function of clotting is to prevent excessive bleeding and promote the healing of damaged tissues. The process involves a series of biochemical reactions that lead to the formation of a blood clot.
Blood is an essential part of the human body, & the defeat of this fluid may be life-threatening. Blood is developed through hematopoiesis & ultimately becomes the delivery method for oxygen to the tissues & cells. The human body defends against blood loss via the clotting mechanism. Vascular mechanisms, platelets.
Prostaglandins, enzymes, coagulation factors, & proteins are the supporters of the clotting mechanism which work together to form clots & stop blood loss. Through vasoconstriction, adhesion, activation, & aggregation, the contributors form a transient plug to work as the cork to the leaking of the flow of blood. After, fibrin, the functioning condition of fibrinogen, stabilizes this inadequate platelet plug.
Cellular Level
The cellular elements of the clotting mechanism contain platelets, endothelial cells, & a series of proteins, enzymes, & ions.
Organ Systems Involved
The clotting mechanism affects the circulatory system which contains the lineage of blood cells & blood vessels.
Mechanism
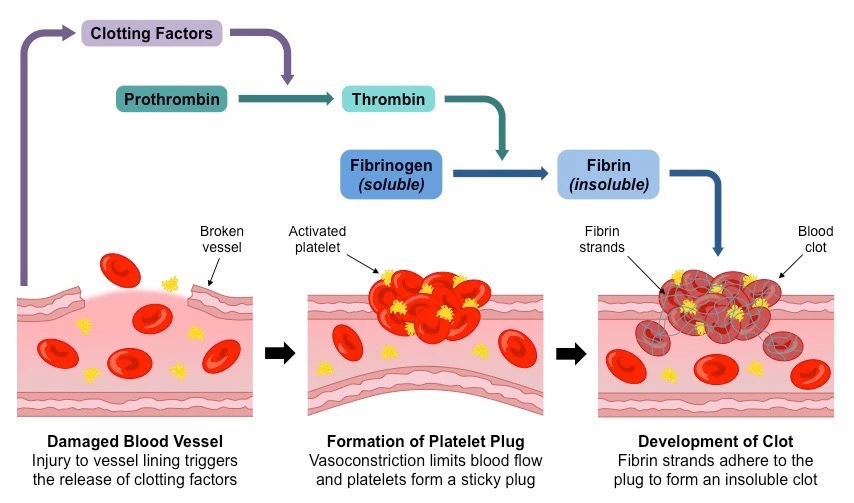
The clotting mechanism is broken into 2 phases:
- Primary hemostasis: Building of a weak platelet plug
- Secondary hemostasis: Stabilizing the inadequate platelet plug into a clot by the fibrin network.
Primary Hemostasis
Primary hemostasis is the appearance of a weak platelet plug which is conducted in four phases: vasoconstriction, platelet adhesion, platelet activation, & platelet collection.
Vasoconstriction is the starting response whenever there is vessel damage. Vasospasm of the blood vessels happens 1st in response to damage to the vasculature. This vasospasm, in turn, produces vasoconstriction. Vasoconstriction is primarily judged by endothelin-1, a potent vasoconstrictor, which is synthesized by the injured endothelium. Damaged endothelium reveals sub-endothelial collagen, von Willebrand factor [ vWF ], and releases ATP, and inflammatory mediators. von Willebrand factor is synthesized by megakaryocytes which subsequently get stored in a-granules of platelets. Weibel palate bodies are the variety of directions of vWF, subendothelial collagen, ATP, & inflammatory mediators which provide the gateway into the 2nd phase of primary hemostasis, platelet bonding.
Platelet adhesion is the method by which platelets connect to the exposed subendothelial vWF. Platelets begin to roll along vessel walls & connect to areas of uncovered subendothelial collagen & vWF. Platelet membranes are rich in G protein [ Gp ] receptors discovered within the phospholipid bilayer. Especially, it is the Gp Ib-IX receptor on platelets that attach to vWF within the endothelium that forms the starting connection between the two. Once bound, a combination of events can occur in the 3rd phase of primary hemostasis to start the platelet.
Platelet activation consists of platelets participating in 2 specific events once they have connected to the exposed vWF [ that means the damaged vessel site ]. Second, platelets secrete their cytoplasmic granules.
Platelet activation is intervened via thrombin by 2 mechanisms. Thrombin directly triggers platelets through proteolytic division by binding the protease-activated receptor. Thrombin also stimulates platelet granule release which contains serotonin, platelet-activating factor, & Adenosine Diphosphate [ ADP ]. ADP is an essential physiological agonist which is stored especially in the dense granules of platelets. When ADP is removed, it binds to P2Y1 & P2Y12 receptors on platelet membranes. P2Y1 induces the pseudopod shape change & aids in platelet aggregation. P2Y12 recreates a major role in causing the clotting cascade. When ADP binds to its receptors, it causes Gp IIb/IIIa complex expression at the platelet membrane externals. It is a calcium-dependent collagen receptor that is essential for platelet-to-endothelial adherence & platelet-to-platelet aggregation. Simultaneously, platelets synthesize Thromboxane A2 (TXA2). TXA2 further strengthens vasoconstriction & platelet aggregation the next step in the primary hemostasis technique. The process of platelet activation trains the local environment for platelet accumulation.
Platelet aggregation starts once platelets have been started. Once triggered, the Gp IIb/IIIa receptors adhere to vWF & fibrinogen. Fibrinogen is located in the circulation & forms a relation between the Gp IIb/IIIa receptors of platelets to interconnect them with each other. This eventually forms the weak platelet plug.
Eventually, primary hemostasis allows the culmination of a weak platelet plug to temporarily shield from hemorrhage until further stabilization of fibrinogen to fibrin via thrombin appears in secondary hemostasis.
Secondary Hemostasis
It affects the clotting factors acting in a flood to eventually stabilize the weak platelet plug.
This is achieved by completing three tasks:
- Triggering activation of clotting factors,
- Conversion of prothrombin to thrombin,
- Conversion of fibrinogen to fibrin.
These studies are accomplished originally by 1 of 2 pathways: the extrinsic & intrinsic pathways, which converge at the activation of factor X & then complete their tasks through the typical pathway. Please note that calcium ions are needed for the whole process of secondary hemostasis. The extrinsic pathway contains tissue factor [TF] and factor VII [FVII]. It is formed when TF binds to FVII, activating FVII to factor VIIa [FVIIa], forming a TF-FVIIa complex. This complex, in turn, triggers factor X [FX]. Note, the TF-FVIIa complex can activate factor IX of the intrinsic pathway, which is called the alternate pathway. One factor X is started to FXa by the TF-FVIIa complex, the cascade restarts down the common pathway. The intrinsic pathway contains the Hageman factor [FXII], factor I [FXI], factor IX [FIX], and factor VIII [FVIII]. The process is started when FXII comes into contact with sensitive subendothelial collagen & becomes activated to FXIIa. Thereafter, FXIIa activates FXI to FXIa, & FXIa activates FIX to FIXa. FIXa works in variety with activated factor VIII [FVIIIa] to trigger factor X. The FIXa-FVIIIa complex first activates Factor X, and then the cascade proceeds down the shared route. The typical pathway originated via the activation of Factor Xa. Factor Xa combines with Factor Va & calcium on phospholipid surfaces to create a prothrombinase complex ultimately activating prothrombin into thrombin. This activation of thrombin occurs through serine protease cleaving of prothrombin. Now, thrombin activates factor XIIIa [FXIIIa]. FXIIIa crosslinks with fibrin including the stabilized clot.
Pathophysiology
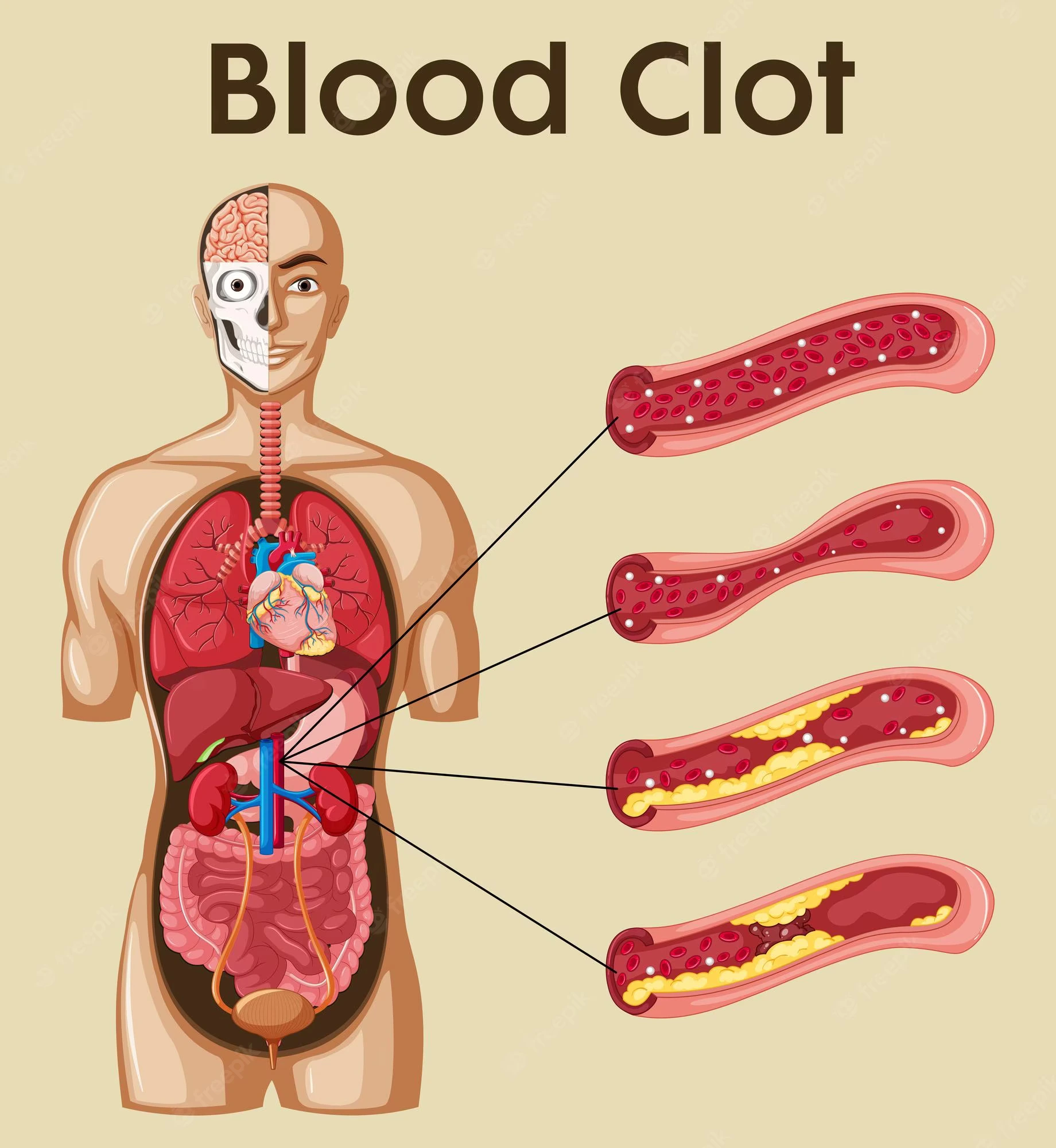
Thrombosis is the procedure of blood clot [ thrombus ] building in a blood vessel. Virchow triad is an essential concept that highlights the primary anomalies in pathology that can cause the clotting mechanism to proceed to thrombosis. The triad is comprised of stasis or turbulent blood flow, endothelial damage, & hypercoagulability of the blood.
Stasis (Abnormal) or turbulent blood flow can cause thrombosis. The normal flow of blood is laminar. Turbulent blood flow cause to endothelial damage thus promoting the formation of a thrombus. Venous stasis, which can result from prolonged bed rest following surgery, long-distance driving or flying, or being overweight and unable to move around, is another factor in irregular blood flow. This damage to the endothelium encourages thrombosis.
Endothelial damage causes platelet activation & the formation of a thrombus. This may be a consequence of inflammation of the endothelial surface of the vasculature. Hypercholesterolemia is an illustration of a chronic inflammatory disease that progresses into endothelial damage.
Thrombophilia (Hypercoagulability) is any disease of the blood that predisposes a person to thrombosis. This may be a consequence of inherited clotting disorders like a Factor V Leiden mutation or an accepted clotting disorder like transferred intravascular coagulation. Hemorrhage happens when blood escapes from the vessel walls. Platelet dysfunction, or clotting factor dysfunction, can be further broken down into which parts of the clotting mechanism physiology are involved.
Diseases of Primary Hemostasis: vWF, Platelet defects, or Receptor Interference
- Von Willebrand Factor disorder
- Bernard-Soulier disorder
- Glanzmann thrombasthenia
- Medication-induced
Diseases of Secondary Hemostasis: Clotting Factor Defects
- Factor V Leiden
- Vitamin K deficiency
- Hemophilia
- Anti-phospholipid antibody syndrome
- Disseminated intravascular coagulation
- Liver disease
- Medication-induced
Defects in Small Vessels
- Trauma
- Aneurysm rupture
- Vasculitides
Clinical Significance
In addition to the pathophysiology, many ideas to keep in mind when having a patient with clotting mechanism diseases:
Patients with:
Primary hemostasis faults generally present with small bleeds in the skin and mucosal membranes. This contains petechiae and purpura.
Secondary hemostasis faults are typically present with bleeds into soft tissue [ muscle ] or joints [ hemarthrosis ].
Direct faults in small blood vessels normally present with palpable purpura & ecchymosis. These may collect & become larger to form a hematoma.
Also, laboratory testing affecting PTT or PT/INR can be separated by the physiological mechanisms:
Diseases only affecting primary hemostasis do not impact the PT or INR or PTT, they only increase bleeding time.
Diseases that affect the extrinsic pathway of secondary hemostasis affect the PT or INR.
Diseases that impact the intrinsic pathway of secondary hemostasis influence PTT.
FAQ
Constriction of the blood vessel. 2. Formation of a temporary “platelet plug.” 3. Activation of the coagulation flood. 4. Construction of the final clot or fibrin plug.
During menstruation, the hormones in the body cause the lining of the uterus to begin shedding. During that process, small blood vessels bleed. To avert the body from losing too much blood, plasma & platelets work together to develop blood clots.
Black blood can appear at the start or end of a person’s period. The color is generally a sign of old blood or blood that has taken longer to exit the uterus & has had time to oxidize, first turning brown or dark red & then ultimately becoming black.
The intermediate time range for blood to clot is about 10-13 seconds. A number higher than that range means it brings blood longer than usual to clot. A number lower than that range represents blood clots more fast than normal.
Thin and Watery
If the blood becomes thinner or watery a watery discharge of any color, could be a manifestation of anemia or a tumor. If the period blood gets thinner over 2 or 3 cycles, about being tested for nutritional deficits or a fallopian tumor.