Facioscapulohumeral Muscular Dystrophy (FSH, FSHD)
Table of Contents
What is facioscapulohumeral muscular dystrophy?
Facioscapulohumeral muscular dystrophy (FSHD) is a genetic muscle disorder that includes muscles of the face, shoulder blades, and upper arms that are the most affected.
Muscular dystrophy means progressive degeneration of muscles, with increased weakness of the muscle and loss of muscle bulk (atrophy). In this type of muscular dystrophy, the first weakness then seriously affects the other portions like; the face, shoulders, and upper arms. Moreover, this disease also causes weakness in other muscles. Facioscapulohumeral muscular dystrophy is the third most common type of muscular dystrophy after the Duchenne and Becker muscular dystrophy and myotonic dystrophy.
Causes of Facioscapulohumeral Muscular Dystrophy:
Genetic:
The genetics of FSHD is complex. FSHD and myotonic dystrophies have special genetic systems that vary from the rest of other genetic myopathies. The DUX4 gene is the main point of FSHD genetics. Normally, DUX4 is demonstrated during embryogenesis and later suppressed in all tissues except the testes. In FSHD, there is the failure of DUX4 suppression and continued production of DUX4 protein, which is toxic to muscles. The mechanism of failed DUX4 suppression is hypomethylation of DUX4 and its surrounding DNA on the tip of chromosome 4 (4q35), which allows for the transmission of DUX4 into messenger RNA (mRNA).
Several mutations can result in disorder, upon which FSHD is further classified into FSHD type 1 (FSHD1) and FSHD type 2 (FSHD2). This disorder can only result when a mutation is present combined with selected, commonly found differences of 4q35, termed haplotype polymorphisms. There are 17 4q35 haplotype polymorphisms, approximately dividable into the groups 4qA and 4qB. A 4qA haplotype polymorphism, known as a 4qA allele, is necessary for this disorder, as it contains a polyadenylation sequence that allows DUX4 mRNA to resist degradation long enough to be translated into DUX4 protein.

DUX4 and the D4Z4 repeat array:
DUX4 inhabits the D4Z4 microsatellite repeat array, a sequence of tandemly repeated DNA segments in the subtelomeric region (4q35) of chromosome 4. Each D4Z4 repeat is 3.3 kilobase pairs (kb) long and is the site of epigenetic regulation containing both heterochromatin and euchromatin formations. In FSHD, the heterochromatin structure is wasted, becoming euchromatin.
The subtelomeric region of chromosome 10q includes a tandem repeat structure extremely homologous (99% identical) to 4q35, containing “D4Z4-like” repeats with protein-coding zones identical to DUX4 (D10Z10 repeats and DUX4L10. Because 10q generally lacks a polyadenylation sequence, it is usually not implicated in the disorder. However, chromosomal reallocation can occur between 4q and 10q repeat arrays, and involvement in disease is possible if a 4q D4Z4 repeat and polyadenylation signal are transferred onto 10q, or if rearrangement causes FSHD1.
FSHD1:
FSHD includes deletion of D4Z4 repeats (termed ‘D4Z4 contraction’) on 4q and is classified as FSHD1, which reports for 95% of FSHD cases. Typically, chromosome 4 involves 11 and 150 D4Z4 repeats. In FSHD1, there are 1–10 D4Z4 repeats. The number of repeats is approximately conversely associated with disease severity. With 8 – 10 repeats tend to have minimal exhibits, sometimes with no symptoms; those with 4 – 7 repeats have a modest disorder that is highly different; and those with 1 – 3 repeats are more likely to have severe and early-onset disorder deletion of the whole D4Z4 repeat array does not result in FSHD because then there are no any copies of DUX4 to be shown, although other birth defects result.
It has been suggested that FSHD1 experiences anticipation, a trend primarily associated with trinucleotide repeat disorders in which the disorder indicates deterioration with each subsequent generation. If anticipation occurs in FSHD, the mechanism is different with the trinucleotide repeat disorders, since D4Z4 repeats are much larger than trinucleotide repeats, an underabundance of repeats (rather than overabundance) causes disease, and the repeat array size in FSHD is stable across generations. D4Z4 array examples, with each D4Z4 repeat described by a triangle.
FSHD2:
FSHD without D4Z4 contraction is classified as FSHD2, which composes 5% of FSHD cases. Different mutations can cause FSHD2, all resulting in D4Z4 hypomethylation, in which the genetic mechanism approaches FSHD1. Approx 80% of FSHD2 cases are due to disconnect mutations in the gene SMCHD1 (structural maintenance of chromosomes flexible hinge domain containing 1) on chromosome 18. SMCHD1 is reliable for DNA methylation and its deactivation results in hypomethylation of the D4Z4 repeat array. Another cause of FSHD2 is a mutation in DNMT3B (DNA methyltransferase 3B), which may also play an important role in DNA methylation.
Mutation of a single allele of SMCHD1 or DNMT3B can cause this disorder. Mutation of both copies of LRIF1 has been tentatively showing a cause of this disorder in a single person as of 2020. As in FSHD1, a 4qA allele must be present for the disease to result. However, in the D4Z4 array, the genes involved in FSHD2 are not in proximity with the 4qA allele, and so they are generic independently from the 4qA allele, resulting in a genetic inheritance pattern.
Pathophysiology:
Molecular:
There appears to be a concurrence that an abnormal manifestation of DUX4 in muscle is the reason for FSHD. DUX4 is manifested in very small amounts, identified in 1 out of each 1000 immature muscle cells (myoblast), which is shown to extend after myoblast maturation, partly because the cells combine as they mature, and one nucleus expressing DUX4 can provide DUX4 protein to neighboring nuclei from merge cells.
It remains a neighborhood of active research on how DUX4 protein causes muscle damage. DUX4 protein may be a transcription factor that controls many other genes. A number of these genes are involved in apoptosis, like p53, p21, MYC, and ß-catenin, and indeed, it appears that DUX4 protein makes muscle cells more susceptible to apoptosis. Other DUX4 protein-regulated genes are involved in oxidative stress, and indeed, it seems that DUX4 expression lowers muscle fiber tolerance to oxidative stress. Differentiation within the ability of individual muscles to handle oxidative stress could partially explain the muscle involvement patterns of FSHD. DUX4 protein down-regulated many genes involved in muscle development, including MyoD, myogenin, desmin, and PAX7, and indeed DUX4 expression has been shown to cut back vegetative cell proliferation, fusion, and differentiation. DUX4 protein regulates some genes that are involved in RNA internal control, and indeed DUX4 expression has been shown to cause the buildup of RNA with subsequent apoptosis. Estrogen seems to play a task in modifying DUX4 protein effects on muscle differentiation, which could explain why females are lesser affected than males.
Muscle histology:
Cross-sectional in microscopic shows affected muscle fibers of FSHD. Also seen are inflammation and fibrosis, similarly to muscle fiber’s shape change, death, and regeneration. In other muscular dystrophies, early muscle biopsies show only cell hypertrophy, displacement of nuclei from myofiber peripheries (central nucleation), and moderate degrees of fibrosis. More often found is inflammation. There’s often endomysial inflammation, first composed of CD8+ T-cells, although these cells don’t directly cause cell death. Endomysial blood vessels can even be found by inflammation, which is comparatively unique to FSHD, and this inflammation contains CD4+ T-cells. Inflammation is succeeded by deposition of fat (fatty infiltration), then fibrosis. Muscle fibers can show whorled, moth-eaten, and, especially, lobulated.
Muscle involvement pattern:
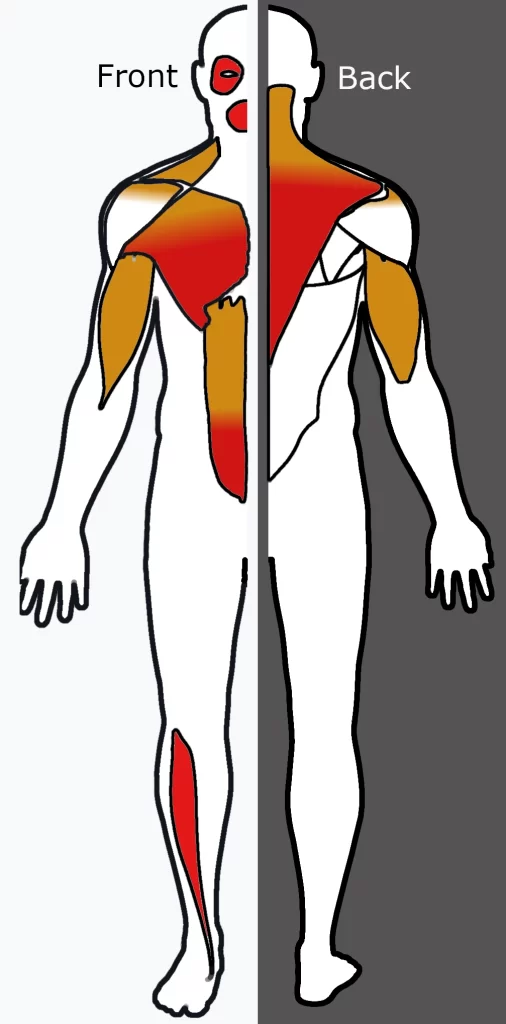
Some muscles are preferentially affected by FSHD, which remains unknown. There are multiple trends of involvement that are seen in FSHD, possibly hinting at underlying pathophysiology. Individual muscles can weaken while adjacent muscles remain healthy. The correct shoulder and arm muscles are more often affected than the left upper extremity muscles, a pattern also seen in Poland syndrome and hereditary neuralgic amyotrophy; this might reflect a genetic, developmental/anatomic, or functional-related mechanism. The deltoid is often spared, which isn’t seen in the opposite condition that affects the muscles around the scapula.
Medical imaging like (CT and MRI) have shown muscle involution
- A single MRI study shows the teres muscle to be commonly affected.
- The semimembranosus muscle, a part of the hamstrings, is often affected, deemed by one author to be “
- he most often and severely affected muscle.”
- Of the quadriceps muscles, the rectus femoris is affected
- Of the gastrocnemius, the medial section is affected;
- The iliopsoas, a hip skeletal muscle, is extremely spared.
Symptoms of FSHD
Symptoms usually show up during their teenage years. But sometimes FSHD appears in babies. The primary belongings you may notice are weakness in a child’s face and shoulder muscles. FSHD affects one side of the body to the opposite. FSHD rarely affects the center and system. It also tends to get worse slowly. The severity of FSHD varies lots, but the majority of the disease have a standard life.

Common symptoms include:
- Scapular winging
- The trouble with reaching up with the arms and throwing a ball
- Difficulty whistling, representing a balloon, or employing a straw
- Difficulty with the mouth cornered up when smiling
- Eyes not closing all the way during sleep
Weakness in lower limb muscles like ankle or pelvic muscles makes it difficult to steer.
Classically, weakness develops within the face, then the pectoral arch, then the upper arm. These muscles may be spared and other muscles are usually affected. The order of muscle involvement can cause the aspect of weakness “descending” from the face to the legs. The distribution of muscle weakness is extremely changeable even between identical twins. Musculoskeletal pain is extremely common, most frequently described within the neck, shoulders, lower back, and therefore the back of the knee. Fatigue is additionally common. Muscle weakness usually becomes noticeable on one side of the body before the opposite, an indicator of the disease. The correct shoulder and arm muscles are more often affected than the left upper extremity muscles, independent of handedness.
Face
Weakness of the muscles of the face is the most distinctive sign of FSHD. It’s typically the earliest sign, although it’s rarely the initial complaint. A minimum of mild facial weakness is found in 90% or more with FSHD. One of the foremost common deficits is the inability to shut the eyelids, which may end in sleeping with the eyelids open and dry eyes. The implicated muscle is the orbicularis oculi muscle. Another common deficit is an inability to purse the lips, causing an inability to pucker, whistle, or increase a balloon. The implicated muscle is the orbicularis oris muscle. A 3rd common deficit is the inability to raise the corners of the mouth, causing a “horizontal smile,” which looks more sort of a grin. The responsibility is the zygomaticus major muscle.
Weakness of assorted facial muscles contributes to difficulty pronouncing the letters M, B, and P.[citation needed] Facial expressions can appear diminished, arrogant, grumpy, or fatigued. Muscles used for chewing and moving the eyes aren’t affected. Difficulty swallowing isn’t typical, although it can occur in advanced cases. FSHD is mostly progressive, but it’s not established whether the facial weakness is progressive or stable throughout life.
Shoulder, chest, and arm
Bilateral scapula winging, after the facial weakness, weakness usually develops within the muscles of the chest and wingers that span from the scapula to the thorax. Symptoms involving the shoulder, like difficulty working with the arms overhead, are the initial complaint in 80% of cases. The serratus magnus and middle and lower trapezii muscles are affected and the upper trapezius is spared. Trapezius weakness causes the scapula to become downwardly rotated and protracted, leading to the winged scapula, horizontal clavicles, and sloping shoulders; arm abduction is impaired. Serratus muscle weakness impairs arm flexion, and worsening of scapula winging will be demonstrated when pushing against a wall. Muscles spanning from the scapula to the arm are generally spared, which include the deltoid and also the structure muscles. The deltoid is often affected for a while, especially the upper portion.
Muscle wasting can make bones and spare shoulder muscles visible, an example being the “poly-hill” sign shown by arm elevation. The first bump is that the upper corner of the scapula appears to “herniate” up and over the body structure. The second hill is the AC joint. It is seen between a wasted upper trapezius and the upper deltoid. The third hill is the lower deltoid, distinguishable between the wasted upper deltoid and humeral muscles. Shoulder weakness and pain can cause shoulder instability, like recurrent dislocation, subluxation, or downward translation of the humeral head.
Also affected is the chest, particularly the parts of the pectoralis muscle that connect with the sternum and ribs. The part that connects to the clavicle is a small amount often affected. This muscle wasting pattern can contribute to a horizontal anterior axillary fold. The forearms are usually spared, leading to an appearance of some compare to the character Popeye, although when the forearms are affected in advanced disease, the wrist extensors are more often affected.
Lower body and trunk
After the upper body, weakness can appear in either the pelvis or it “skips” the pelvis and involves the tibialis anterior (shin muscle), causing the foot to drop. The pelvic and thigh muscles are the last group affected. Pelvic muscle weakness can show as pelvic tilt, causing the hips to be maintained in slight flexion. Pelvic weakness can also cause a Trendelenburg sign. Weakness of the back of the thigh (hamstrings) is more common than the weakness of the front of the thigh (quadriceps).
Weakness also occurs in the abdominal muscles and paraspinal muscles, which can manifest as a protuberant abdomen and lumbar hyperlordosis. Abdominal weakness can cause the inability to do a sit-up, and also the inability to turn from one side to the other while lying on one’s back. Of the rectus abdominis muscle, the lower portion is affected, it is manifesting as a positive Beevor’s sign. The neck extensor weakness can cause the head to lean towards the chest, termed head drop.
The most common non-musculoskeletal manifestation of FSHD is abnormalities within the small arteries (arterioles) within the retina. The sinuosity of the arterioles is seen in approximately 50% of those with FSHD. Less common arteriole abnormalities include telangiectasias and microaneurysms. These abnormalities of arterioles usually don’t affect vision or health, although a severe sort of it mimics Coat’s disease, a condition found in about 1% of FSHD cases and more frequently related to large 4q35 deletions. High-frequency deafness can occur in those with large 4q35 deletions, but otherwise, it isn’t any more common compared to the final population.
Scoliosis may also occur, the result of the weakness of the abdominal, hip extensor, and spinal muscles. In contrast, scoliosis will be viewed as a compensatory mechanism for weakness. Breathing can even be affected by kyphoscoliosis and wheelchair use. It’s seen in one-third of wheelchair-using patients. However, ventilator support is required in a number of cases. Although there are reports of increased risk of cardiac arrhythmias, the general consensus is that the guts aren’t affected.
These can (but don’t always) include:
- Inability to whistle
- Inability to sip through a straw
- Eyes that don’t close fully during sleep
- Difficulty with sit-ups and pull-ups
- Shoulder blades that “wing” out
- Difficulty raising the arm above shoulder height
- Weakness in hands and fingers
- Foot drop (foot dorsiflexion weakness)
- Weak lower abdominal muscles, “pregnant” belly
- Loss of chest (pectoral) muscles
- Sunken breastbone (pectus excavatum)
- Curved spine (lordosis, kyphosis, scoliosis)
- Chronic fatigue
Diagnosis of FSHD:
A diagnosis of facioscapulohumeral hereditary disease (FSHD) is suspected in patients who present with weakness of the face, arch, and upper arm(s) with relative sparing of the deltoid muscles. Patients with the suspected genetic disease should be stated to a specialist expertly in neuromuscular disorders (where available) for evaluation and diagnosis.
The most reliable way to diagnose FSHD is with a test for a small missing section of DNA on chromosome 4. an ad genetic test for FSHD detects shortening of the repeated DNA elements (D4Z4) located within the 4q35 region of chromosome 4. This assay, performed on blood cells, is positive in approximately 95% of typical FSHD cases. The test is highly accurate for FSHD.
Genetic testing: it’s not needed for each affected person with a typical clinical presentation if the case history is in line with autosomal dominant inheritance and also the diagnosis has been genetically confirmed by a first-degree relative. In many cases, however, people with no case history are suspected of getting either FSHD or another neuromuscular disorder. In these situations, less costly and fewer specific tests than the FSHD DNA test are also done first.
Creatine kinase level: This test, also performed on a blood sample, measures the quantity of an enzyme called creatine kinase within the blood. When muscle cells break down, as they are doing in muscular dystrophies and a few other disorders, the creatine kinase, or CK, the level is elevated. CK is commonly elevated up to five times the upper limit of normal in symptomatic FSHD patients. CK isn’t elevated in patients with no symptoms.
Electromyogram or EMG: which measures the electrical activity within the muscles, displayed within the sort of waves. EMG typically displays alterations in patients with FSHD.
Muscle biopsy: During this procedure, a little piece of muscle is taken, under anesthesia, usually from the arm or leg.
Differential Diagnoses:
- Amyotrophic Lateral Sclerosis in Physical Medicine and Rehabilitation
- Congenital Muscular Dystrophy
- Congenital Myopathies
- Diabetic Neuropathy
- Emery-Dreifuss Muscular Dystrophy
- Endocrine Myopathies
- Inclusion Body Myositis
- Inherited Metabolic Disorders
- Physical Medicine and Rehabilitation for Limb-Girdle Muscular Dystrophy
- Polymyositis
Treatment of FSHD
Medical Management
Medical treatments for facioscapulohumeral genetic disorders (FSHD) are relatively few, and none are specific to the disease. there’s no treatment that will halt or reverse the results of FSHD, but there are few treatment options and devices to alleviate many symptoms.
Anti-inflammatory drugs called nonsteroidal anti-inflammatories, or NSAIDs, are often prescribed to enhance comfort and mobility. These are the identical drugs taken by many folks with arthritis and other inflammatory conditions. Antidepressants or antiepileptics are appropriate for chronic pain.
Surgical and mechanical help
Surgical procedures help to stabilize the shoulder blades (scapulae) by attaching them to the ribs have helped some people with FSHD. During this procedure, the scapulae are fixed to the ribs in order that they are doing not move. The patient gains some advantage with the arm on the side that’s had the operation because the scapulae do not slide around. This kind of surgery may fine reduce the range of motion of the arm (because the scapula can’t rotate normally), the flexibility of the arm to function is additionally better because the arm’s flexibility point is now stable.
It is important to excursion a surgeon who fully understands FSHD and has had experience with this exact sort of surgery.
Surgical scapular fixation
A recent review terminated that surgical scapular fixation is useful in improving shoulder function in FSHD. this is often confirmed in reviews of more modern case series. Scapulodesis, the fixation of the scapula with screws, wires, or plates with bone grafting (arthrodesis), is the preferred surgery and may be performed by an experienced surgeon. Patients considering surgery should have the reasonable unused upper arm strength and may consider the potential benefits against the possible complications of the procedure. Potential surgical or post-surgical complications include breaks within the wire with consequent loss of functional gain and, rarely, nerve plexus injuries. The potential gain in the range of motion from surgical fixation is tested at the bedside by manual fixation of the scapula. the correct indications for the procedure in FSHD haven’t been probably uncompromising.
Physiotherapy Treatment for Facioscapulohumeral muscular dystrophy
Role of physiotherapy and rehabilitation
It is recommended that every patient with FSHD who have functional limitations get an initial rehabilitation consult. Such consultation may address functional limitations including assessment of balance and gait, posture, and therefore the need for orthoses. Complaints of fatigue and pain must be specifically addressed likewise. Recommendations regarding an appropriate exercise regimen including stretching, resistive and aerobic training may be provided to support the therapy evaluation and also the current evidence for exercise in FSHD. Follow-up physiatrics evaluations will rely upon the continuing needs of individual patients. Patients with mild functional disabilities may require yearly follow-up whereas patients with severe infantile-onset disease, may require ongoing input from a physiotherapist, orthotist, occupational therapist, and speech pathologist. an in-depth physiatrics brochure for FSHD.
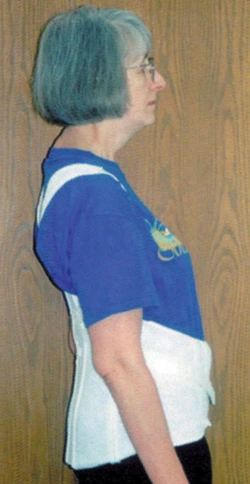
Orthosis
Physical therapists often recommend devices like back supports, corsets, girdles, and special bras for people with FSHD. These supports help to make amends for weakening muscles within the upper and lower back.
Lower leg braces, called ankle-foot orthoses, or AFOs, can atone for weakening muscles within the lower leg that cause tripping and falling. There is also recommended by a physician or therapist and might be purchased as off-the-shelf or custom-made models. Some people find a lightweight, the high-top shoe may be as helpful as an AFO in supporting the foot, a minimum of within the early stages of weakness.
A corset-style back support tired under clothes can help keep the shoulders and lower back in better alinement.
Physiotherapists advise that those with FSHD shouldn’t resist using these variations of devices for fear their muscles will get “lazy.” A supportive corset or AFO can help with mobility and endurance, they say, and supporting muscle during a normal position can facilitate your use of your remaining strength more effectively.
Massage or warm, moist heat (for example, from hot packs you’ll be able to put in a very microwave) are good for the discomfort related to FSHD. Patients with severe early-onset types of FSHD may like physical, occupational, and therapy.
Facial Muscle Weakness
An inability to smile due to skeletal muscle weakness remains a significant social disability in FSHD. Weakness of the labial muscles can result in articulation difficulties within the later stages but doesn’t achieve the purpose at which alternative means of communication become necessary. On the premise of their finding of swallowing difficulties, Wohlgemuth et al33 recommended that patients be brought up to speech therapists for further evaluation of and teaching associated with compensatory strategies for swallowing. Weakness of the orbicularis oculi can cause incomplete lid closure, which may result in serious exposure to keratitis and corneal scarring, major safety concerns. Eye drops, ointments, taping, or patches haven’t always been successful in managing these problems.
Pain and fatigue
Pain could be a common and underestimated complaint in FSHD. Using the McGill pain questionnaire and a self-observation list on daily observed pain prospectively, the pain was present in 77% of a gaggle of 79 FSHD patients. An unpublished survey by the AFM (Association Française Contre less Myopathies) showed that 55% of FSHD patients complained of pain a minimum of several days every week. more modern studies have also documented the effect of pain on patients with FSHD. The etiologies of the pain are multiple and will be proscribed using standard approaches to the management of chronic pain like, where appropriate, therapy and pain medications. Fatigue is additionally a frequent complaint, is multifactorial in origin, and is experienced by patients with a variety of dystrophies. Energy maintenance strategies can help some patients as aerobic training. It’s maintenance noting that mood disorders although not present at the next periodicity than within the normal population, can increase both symptoms of pain and fatigue and will be appropriately addressed when present.
Respiratory dysfunction
Clinically significant respiratory insufficiency occurs in less than 1% of patients with FSHD. Nevertheless, clinicians must remain vigilant as compensated respiratory insufficiency is also unmasked by medical stressors. it’s recommended that patients with moderate to severe FSHD, defined as those with proximal lower extremity weakness, be routinely screened for symptoms of hypoventilation. Measurement of supine and sitting forced diagnostic assay (FVC) is suggested for any patient with FSHD before any surgical operation requiring anesthesia or conscious sedation. Yearly FVC is usually recommended for all patients who are wheelchair bound, have pelvis weakness and superimposed pulmonary disease, and have moderate to severe kyphoscoliosis or lumbar hyperlordosis or chest wall deformities like pectus excavatum. FVC measurements in patients with FSHD should be performed with a full facial mask instead of a mouthpiece to avoid measurement of falsely low values from air leakage thanks to the weakness of lip closure. Signs and symptoms of nighttime hypoventilation or a drop of FVC to but 50% of predicted value warrant the consideration of non-invasive ventilatory support devices like BiPAP.
Cardiac dysfunction
Cardiac involvement in FSHD manifested as a predilection to atrial arrhythmias is seen in about 5% of patients without other cardiac risk factors, few of whom require treatment. the foremost common ECG finding is an increased frequency of RBBB which is usually of no clinical importance. there are insufficient data to warrant routine ECG screening on all patients with FSHD. The presence of clinically significant cardiac dysfunction should cause the consideration of other diagnoses.
Pregnancy in FSHD
Pregnancy outcomes in FSHD are generally good although two case ranges have contradictory reports about an increased incidence of functional deliveries and preterm births. a number of the differences are also thanks to differences in obstetrical practice within the countries where the studies were conducted. Resolving this discrepancy necessitates further prospective studies. additionally, about 25% of pregnant women with FSHD report subjective, persistent worsening of mo
formation, it’s suggested that pregnant women with FSHD be followed by high-risk obstetricians whose delivery occurs in an exceedingly center which will provide comprehensive perinatal care. Additionally, it’s recommended that pregnant women with FSHD and reduced lung function have serial monitoring of their FVC during the course of their pregnancy.
Hearing loss
Subclinical hearing disorder in FSHD occurs in up to 75% of affected individuals but the frequency isn’t different from an effect population. Infantile-onset patients with FSHD are in danger to own the foremost profound deafness that if not detected can result in delayed language development and even the false perception that the kid is cognitively delayed. In contrast, it should be tested regularly in infants and preschool children diagnosed with FSHD. Older-aged children diagnosed with FSHD don’t need an audiogram if their hearing is regularly tested at school and if they demonstrate normal language development. Adults diagnosed with FSHD don’t require audiograms unless they’re symptomatic.
Abdominal Muscle Weakness and Lordosis
There aren’t any reports within the literature of specific management techniques that are useful for improving posture or decreasing discomfort secondary to a postural abnormality in patients with FSHD. Clinicians tend to recommend abdominal supports and binders for patients with marked weakness and appropriate postural supports and environmental adaptations when patients are seated.
Ankle Muscle Weakness
Foot drop owing to ankle dorsiflexor muscle weakness may be a common problem in patients with FSHD. There are not any reports within the literature of the precise kinetic or kinematic patterns of gait seen in patients with FSHD to guide management. There are also no reports regarding algorithms for the timing or choice of specific sorts of ankle-foot orthoses and no reports regarding the advantages of those orthoses in patients with FSHD with respect to gait or functional abilities. Decisions regarding the timing and sort of orthoses are supported by empirical data and vary from clinician to clinician.12–14 The braces most ordinarily recommended include fixed or hinged ankle-foot orthoses or floor reaction orthosis.
Exercise for Facioscapulohumeral muscular dystrophy
Because the precise underlying defect that causes muscle loss in FSHD isn’t yet understood, it’s hard to create precise recommendations about exercise. However, physical therapists who have observed people with FSHD for several years say that moderate exercise appears to try to do no harm and will even be helpful, a minimum for muscles that haven’t severely weakened.
Therapists advise that exercise shouldn’t cause muscle cramping, significant muscle pain, or extreme fatigue. An exercise program for somebody with FSHD should be directed by a knowledgeable, physical or occupational therapist, who has experience with neuromuscular disorders. The program should emphasize exercising muscles that are still relatively strong and resting in people who have weakened. this may be accomplished with careful positioning and adaptation of ordinary exercise regimens.
The current recommendations 59–61 are as follows:
pursuit of a lively lifestyle for its physical and psychological benefits
flexibility training including stretching and range-of-motion exercises, which can be helpful in decreasing the discomfort due to the limited mobility of the joints secondary to muscle weakness
moderate-intensity resistive strengthening exercise, which can be beneficial in reversing disuse weakness and improving strength during the first stages of the disorder in muscles that have anti-gravity or greater strength
moderate-intensity aerobic training programs, which can be beneficial in maintaining or improving aerobic capacity.

Aerobic exercise
Aerobic exercise is any exercise that increases the guts rate. For those with inherited diseases, you ought to be able to exercise while holding a conversation. If you can’t talk because you’re too out of breath then you’re doing an excessive amount. the kind of aerobics will rely on your condition, but low-impact exercises like fast walking, dancing, swimming, and cycling are all options.
The Muscular Atrophy News forums are an area to attach with other patients, share tips and speak about the newest research. Check them out today!
Ideally, you ought to exercise 20 minutes every day between four and 6 times per week, but you may want to begin by doing five or 10 minutes each day and building your fitness levels up slowly. make sure you warm up gently before exercising and stretch your muscles well after to decrease the prospect of injury.
Strengthening Exercises
Strength exercises directly target the muscles using either hand-held weights or machines or using your own weight. Because the muscles of somebody with MD are already weak, it’s recommended that they follow a lightweight, high-repetition regimen instead of trying to lift heavy weights. Keep the weights somewhere between five and 10 pounds to start out and once you feel comfortable gradually increase the weights. Strength exercises are done on their own or alongside other kinds of exercise.
Stretching Exercise

Exercises that incorporate stretching like yoga, Pilates, and t’ai chi are great for improving tone also as helping with joint stiffness. If you don’t want to affix a category then there are plenty of online tutorials for stretching which will help your condition. you’ll be able to pick those you’re feeling most comfortable with, then try and hold each stretch for between 10 and 30 seconds. it’s important to warm up before beginning any stretching routine.
Respiratory Techniques:
assisted coughing, diaphragmatic breathing exercises
Complication
- Pain and fatigue
- Facial Muscle Weakness
- Respiratory dysfunction
- Cardiac dysfunction
- Hearing loss
- Abdominal Muscle Weakness and Lordosis
- Ankle Muscle Weakness
- Imbalance
FAQ
What is the anticipation of facioscapulohumeral muscular dystrophy?
FSHD may affect one side of the body quite the opposite. FSHD rarely affects the guts and system respiratory. It also tends to induce worse slowly. The severity of FSHD varies plenty, but the majority of the disease has a traditional life.
Is there a cure for facioscapulohumeral muscular dystrophy?
Presently, the facioscapulohumeral genetic disorder remains incurable. Treatments are given to manage symptoms and improve quality of life. Activity is inspired. Inactivity like bedrest can make muscle disease worse.
What is the primary symptom of facioscapulohumeral muscular dystrophy?
Weakness involving the facial muscles or shoulders is typically the primary symptom of this condition. skeletal muscle weakness often makes it difficult to drink from a straw, whistle or come about the corners of the mouth when smiling.
Does FSHD affect the brain?
A brain volumetric study, for instance, has shown nervous tissue loss in FSHD, especially within the left precentral cortex, the anterior cingulate cortex, and therefore the right frontal region.
What is the expectancy of facioscapulohumeral muscular dystrophy?
FSHD may affect one side of the body quite the opposite. FSHD rarely affects the guts and system. It also tends to induce worse slowly. The severity of FSHD varies plenty, but most people with the disease have a standard era.
How painful is FSHD?
FSHD doesn’t affect sensation, nor does it affect the ability to regulate the bladder and bowels, or sexual function.
Is FSHD dystrophy curable?
There is no available cure for FSHD. Patients are currently managed for their symptoms at their best. While the genetic mechanisms resulting in FSHD are diverse and complicated, these all end in aberrant expression of the double homeobox protein 4 (DUX4) gene in striated muscle.