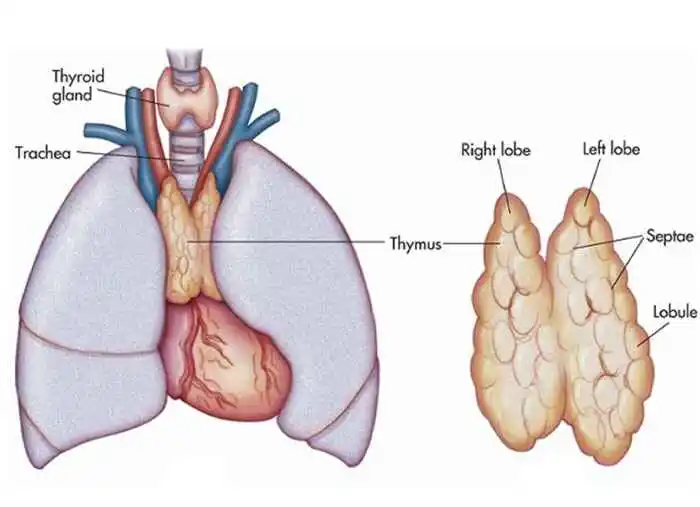
Thymus Gland
Table of Contents
Introduction
The thymus gland is situated beneath your lungs and behind your sternum; it is active until adolescence. The major lymphoid organ for T cell formation and maturation, the thymus, delivers immunological surveillance on tumor cells, immune tolerance to self-antigens, and immune protection against foreign antigens.
The thymus gland is not kept active for the course of a person’s life, but when it does, it plays an important function in assisting the body in responding against autoimmunity, which is the result of the immune system converting against the body. The thymus contributes to the creation of T-lymphocytes, or T cells, which are responsible for protecting the body against pathogens and viruses, both before birth and during childhood.
Immunity
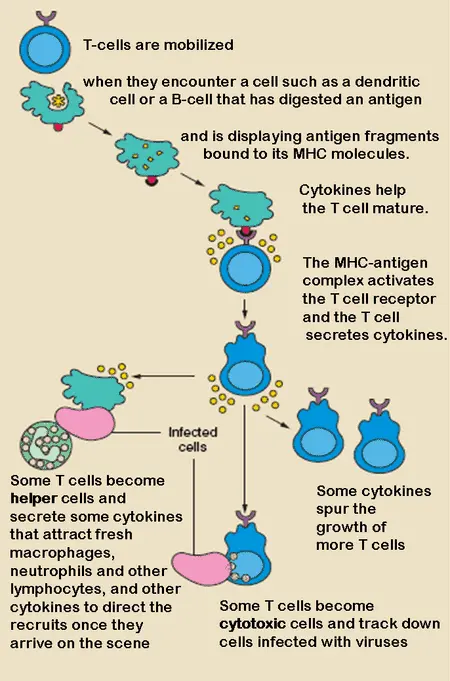
The organ that creates and grows T cells primarily is the thymus, which additionally functions as the starting point for the positive and negative selection events that lead to T cell immunological maturation. T cells obtain their name from the thymus, where they develop, although B cells obtain their name from the bone marrow, where they grow and mature. Both belong to the leukocyte type.
The thymus gland is where T cells increase and initiate transformation into regulatory in nature, helper, memory, or cytotoxic T cells. After that, they circulate by way of the lymphatic or circulatory systems or are transferred to peripheral tissues.
Helper T cells produce cytokines in response to the right antigen, which induces B cells to differentiate into plasma cells (cells that produce antibodies). As their name indicates, regulatory T cells modulate immunological responses. Activated by different cytokines, cytotoxic T cells attach to cancerous and infected cells and destroy them.
Injury
Thymic injury and degeneration can result from small injuries such as infections, chemotherapy, and radiation therapy, as well as changes in the external environment because the thymus gland cells are delicate and sensitive to these processes. Though the thymus gland can repair itself, this ability often proves insufficient to fully restore thymic function. Autoimmunity, continuous tumor growth, opportunistic infections, and weak health conditions are all linked to thymic dysfunction.
Malnutrition is an aspect of thymus gland destruction. The thymus is a true indicator of nutrition, and larger thymuses have connections to all anthropometric elements and breastfeeding.
The thymus gland may recover subsequent fibrofatty atrophy at any point in life, in particular after stressful times. Researchers at Monash University have found a way to activate the thymus gland to cause it to expand to its maximum size and begin generating T cells once more. Research studies on cancer patients receiving donated bone marrow have begun after it was shown to be effective in animals.
Thymic Devolution
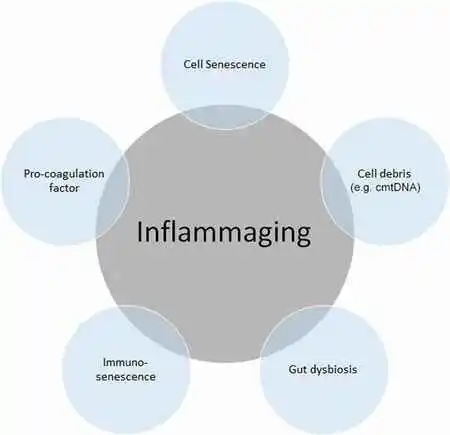
The first sign of immunosenescence and inflammation is thymic devolution. As adults age, their thymus disappears which makes them increasingly susceptible to illness. This decrease in medulla size and function produces an erosion in the overall amount of circulating lymphocytes with the T virus and a change in their function.
This alteration in function may lead to an increase in neoplasms, bacterial and viral infections, and autoimmune disorders. Research on the thymus is currently extremely encouraging as it may be possible to maintain the immune system into adulthood by either reestablishing thymic activity or operating before involution.
Structure
The anterior mediastinum comprises the thymus. It is composed of two lobes that meet in the upper midline and continue down to the fourth rib cartilage from below the thyroid in the neck. There is a capsule protecting the lobes.
The aortic arch and the primary blood vessels divide from the thymus gland, which occupies space on the pericardium in front of the sternum, by a layer of fascia. It can occur for the left brachiocephalic vein to become placed in the thymus.
Function
T cell maturation
T cells are essential parts of the immune system that provide cell-mediated immunity, and the thymus helps them mature. After their maturation process in the thymus, the cells must make sure they respond to antigens (“positive selection”) but not to antigens present on body tissue (“negative selection”). After reaching maturity, T cells leave the thymus to carry out fundamental immune system roles.
Every T cell has an original T cell receptor that is specific to an antigen, which is a particular material. The major histocompatibility complex on tissue cells is connected by the majority of T cell receptors. The T cell receptor receives an antigen from the MHC; if this fits the specific T cell receptor, the receptor becomes activated. Mature T cells are required to be able to destroy antigens that exist in tissues within the body (“negative selection”) and interact with the MHC molecule (“positive selection”).
Positive selection
Specific T cell receptors are present in T cells. The synthesis of these unique receptors results from the rearrangement of the V(D)J recombination gene, which is triggered by the RAG1 and RAG2 genes. Due to the error-prone structure of this process, some thymocytes are unable to produce functional T-cell receptors, whereas other thymocytes produce autoreactive T-cell receptors. The thymocyte will start to display CD4 and CD8, two cell surface proteins, immediately if a functioning T cell receptor has been produced.
Negative selection
The thymus gland technique used to reject T cells aimed at the body’s proteins has been termed as “negative selection.” This is caused by the gene AIRE. Strongly hypersensitive thymocytes to self-antigens do not live and gradually undergo apoptosis.
Blood and nerve supply
The branches could reach the thymus promptly when they emerge, or they may extend beyond the capsule’s septa into the region behind the cortex and medulla. The veins can decrease completely and enter the superior vena cava. Lymphatic vessels connect arteries and veins, but they only move outside of the thymus. These drain into the lymph nodes of the parasternum, tracheobronchial, and brachiocephalic regions. Phrenic nerve branches enter the thymus’s capsule but do not get beyond the actual thymus.
Development
The thymus’s epithelium and thymocytes originate from separate sources. First, to grow, the thymus gland gives rise to two outgrowths, one on either side of the third pharyngeal pouch. In rare cases, the fourth pharyngeal pouch is also affected. These spread both anteriorly to the ventral aorta and laterally into the mesoderm that supports it and the mesenchyme that is derived from the neural crest.
Here, the epithelium and thymocytes approach and unite with connective tissue. Each diverticulum’s pharyngeal hole quickly disappears, but the flask’s neck endures as a cellular cord for a while. The cells lining the flask continue to multiply, developing buds of cells that the creeping mesoderm protects and isolates.
The epithelium gland grows into a structure simulating a sponge and produces microtubules. Hematopoietic bone marrow precursors move into the thymus at this stage. The interactions between the hematopoietic thymocytes and the epithelium must exist for normal development. Thymus growth and activity also need iodine.
Involution
After birth, the thymus keeps growing until puberty, when it reaches its relative maximum size. Fetal and neonatal life takes place when it is most active. By puberty, it has developed to a mass of 20 to 50 grams. After that, during a process known as thymic involution, it starts to shrink in size and activity.
Beyond the first year of life, fewer T cells are generated. It along with connections contribute to just a small part of the lymphatic volume. When involution, the thymus diminishes in size and activity. Fat cells are present from birth, but they become larger and more numerous throughout adolescence. They first infect the gland through the spaces between the lobules, and then they move into the cortex and medulla.
Clinical significance
- DiGeorge syndrome: a congenital (existing from birth) condition where there is inadequate or no thymus. Infants born with DiGeorge syndrome are more open to infections and have severe immunodeficiency or immune system failure.
- Graft-versus-host-disease: An infant with DiGeorge syndrome may benefit from having a thymus gland transplanted from a stillborn baby in an attempt to maybe strengthen their immune system. On the other hand, the recipient’s cells may be attacked by cells from the transplanted thymus.
- Mediastinal masses: Tumors, fluid-filled sacs called cysts, or additional irregularities in your mediastinal organs, such as the thymus, can all be classified as masses. The masses might or might not be malignant.
- Thymoma and thymic carcinoma (thymus cancer): The cells that cover the outside of your thymus can develop into thymoma or thymic carcinoma, two infrequent forms of cancer. Thymomas imitate ordinary thymus cells in appearance, grow slowly, and are frequently confined to the thymus.
- Myasthenia gravis An autoimmune illness where antibodies produced by your immune system inhibit your neurons from sending impulses to your muscles, causing muscular weakness.
- Pure red cell aplasia: a rare autoimmune disease that causes severe anemia by preventing the body from making new red blood cells.
FAQs
What is the thymus’ basic use?
T-lymphocytes, frequently known as T-cells, are a particular kind of white blood cells that the thymus gland develops and trains.
A thymus gland: what is it?
The smallest structure present in the upper chest, over the collarbone’s skin from damage, is the thymus gland. It releases lymphocytes, or white blood cells that protect the body from illnesses.
What is the approximate age of the thymus gland the demise?
The first unequal quality of the thymus is that it shrinks narrower as it is older. After teenagers, the thymus gets started rapidly and by the time we reach age 65, we are incapable of producing new T cells. A process known as involution starts as the organ contracts, causing fatty tissue to replace the T cell regions.
What are thymus gland risk signs?
A cough that doesn’t go away.
Shortness of breath.
Chest pain.
A hoarse voice.
Why do thymus gland troubles occur?
Stress and viral infections may affect your thymus gland. Because the thymus gland lives in your chest, symptoms involving chest pain may appear when damage occurs. Breathlessness and weight loss are other indications that your thymus is damaged.
Which conditions impact the thymus gland?
The three main syndromes tied to thymic ailments are hypogammaglobulinemia, pure red cell aplasia (PRCA), and myasthenia gravis (MG).
References
- Professional, C. C. M. (2024b, May 1). Thymus. Cleveland Clinic. https://my.clevelandclinic.org/health/body/23016-thymus
- Thymus. (2024, September 15). In Wikipedia. https://en.wikipedia.org/wiki/Thymus
- Admin. (2020, August 6). Explore the Meaning of Thymus. Discover Its Role and Function. BYJUS. https://byjus.com/biology/thymus/
- Professional, C. C. M. (2024c, May 1). Thymus. Cleveland Clinic. https://my.clevelandclinic.org/health/body/23016-thymus
- The Editors of Encyclopaedia Britannica. (2024a, September 19). Thymus | Description, Anatomy, & Function. Encyclopedia Britannica. https://www.britannica.com/science/thymus